PDA-TOLERATE Trial: An Exploratory Randomized Controlled Trial of Treatment of Moderate-to-Large Patent Ductus Arteriosus at 1 Week of Age
- PMID: 30340932
- PMCID: PMC6502709
- DOI: 10.1016/j.jpeds.2018.09.012
PDA-TOLERATE Trial: An Exploratory Randomized Controlled Trial of Treatment of Moderate-to-Large Patent Ductus Arteriosus at 1 Week of Age
Abstract
Objective: To compare early routine pharmacologic treatment of moderate-to-large patent ductus arteriosus (PDA) at the end of week 1 with a conservative approach that requires prespecified respiratory and hemodynamic criteria before treatment can be given.
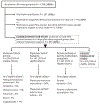
Study design: A total of 202 neonates of <28 weeks of gestation age (mean, 25.8 ± 1.1 weeks) with moderate-to-large PDA shunts were enrolled between age 6 and 14 days (mean, 8.1 ± 2.2 days) into an exploratory randomized controlled trial.
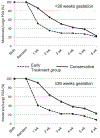
Results: At enrollment, 49% of the patients were intubated and 48% required nasal ventilation or continuous positive airway pressure. There were no differences between the groups in either our primary outcome of ligation or presence of a PDA at discharge (early routine treatment [ERT], 32%; conservative treatment [CT], 39%) or any of our prespecified secondary outcomes of necrotizing enterocolitis (ERT, 16%; CT, 19%), bronchopulmonary dysplasia (BPD) (ERT, 49%; CT, 53%), BPD/death (ERT, 58%; CT, 57%), death (ERT,19%; CT, 10%), and weekly need for respiratory support. Fewer infants in the ERT group met the rescue criteria (ERT, 31%; CT, 62%). In secondary exploratory analyses, infants receiving ERT had significantly less need for inotropic support (ERT, 13%; CT, 25%). However, among infants who were ≥26 weeks gestational age, those receiving ERT took significantly longer to achieve enteral feeding of 120 mL/kg/day (median: ERT, 14 days [range, 4.5-19 days]; CT, 6 days [range, 3-14 days]), and had significantly higher incidences of late-onset non-coagulase-negative Staphylococcus bacteremia (ERT, 24%; CT,6%) and death (ERT, 16%; CT, 2%).
Conclusions: In preterm infants age <28 weeks with moderate-to-large PDAs who were receiving respiratory support after the first week, ERT did not reduce PDA ligations or the presence of a PDA at discharge and did not improve any of the prespecified secondary outcomes, but delayed full feeding and was associated with higher rates of late-onset sepsis and death in infants born at ≥26 weeks of gestation.
Trial registration: ClinicalTrials.gov: NCT01958320.
Keywords: bronchopulmonary dysplasia; necrotizing enterocolitis; newborn; premature birth; retinopathy of prematurity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

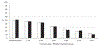

Comment in
-
Is rescue based treatment or early conservative treatment superior in managing patent ductus arteriosus?Acta Paediatr. 2019 Jul;108(7):1363. doi: 10.1111/apa.14809. Epub 2019 Apr 30. Acta Paediatr. 2019. PMID: 31039276 No abstract available.
References
-
- Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at abirth weight of 1000 grams or less. Pediatrics 2006;117:1113–21. - PubMed
-
- Nemerofsky SL, Parravicini E, Bateman D, Kleinman C, Polin RA, Lorenz JM. The ductus arteriosus rarelyrequires treatment in infants >1000 grams. Am J Perinatol 2008;25:661–6. - PubMed
-
- Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, et al. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. J Pediatr 2016;177:66–71.e1. - PubMed
-
- Clyman RI, Mauray F, Heymann MA, Roman C. Cardiovascular effects of patent ductus arteriosus in preterm lambs with respiratory distress. J Pediatr 1987;111:579–87. - PubMed
-
- Shimada S, Kasai T, Konishi M, Fujiwara T. Effects of patent ductus arteriosus on left ventricular output and organ blood flows in preterm infants with respiratory distress syndrome treated with surfactant. J Pediatr 1994;125:270–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

