Varenicline and Bupropion for Long-Term Smoking Cessation (the MATCH Study): Protocol for a Real-World, Pragmatic, Randomized Controlled Trial
- PMID: 30341043
- PMCID: PMC6231835
- DOI: 10.2196/10826
Varenicline and Bupropion for Long-Term Smoking Cessation (the MATCH Study): Protocol for a Real-World, Pragmatic, Randomized Controlled Trial
Abstract
Background: Varenicline and bupropion are efficacious, prescription-only pharmacotherapies for smoking cessation; however, their real-world impact is limited by prescriber knowledge, affordability, and accessibility.
Objective: The primary objective of the MATCH (Medication Aids for Tobacco Cessation Health) study was to evaluate the real-world, long-term effectiveness of mailed bupropion and varenicline in a sample of interested smokers with the utilization of Web-based recruitment and follow-up. In addition, the study aims to investigate the genotypic and phenotypic predictors of cessation.
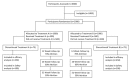
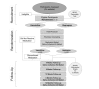
Methods: This is a two-group, parallel block, randomized (1:1) open-label clinical trial. This study will be conducted online with the baseline enrollment through the study's website and follow-up by emails. In addition, medication prescriptions will be filled by the study contract pharmacy and couriered to participants. Individuals who smoke ≥10 cigarettes per day and intend to quit within the next 30 days will be recruited through Public Health Units and Tobacco Control Area Networks throughout Ontario by word-of-mouth and the internet. Eligible participants will receive an email with a prescription for 12-week assigned medication and a letter to take to their physician. The recruitment and randomization will continue until 500 participants per arm have received medication. All participants will receive weekly motivational emails during the treatment phase. The primary outcome measure is the smoking status after 6 months, biochemically confirmed by mailed-in salivary cotinine. Follow-ups will be conducted through emails after 4, 8, 12, 26, and 52 weeks of starting the treatment to assess the smoking prevalence and continuous smoking abstinence. In addition, mailed-in saliva samples will be used for genetic and nicotine metabolism analyses. Furthermore, personality characteristics will be assessed using the Big Five Aspect Scales.
Results: The project was funded in 2014 and enrollment was completed in January 2017. Data analysis is currently underway.
Conclusions: To the best of our knowledge, this is the first randomized controlled trial to mass distribute prescription medications for smoking cessation. We expect this method to be logistically feasible and cost effective with quit outcomes that are comparable to published clinical trials.
Trial registration: ClinicalTrials.gov NCT02146911; https://clinicaltrials.gov/ct2/show/NCT02146911 (Archived by WebCite at http://www.webcitation.org/72CZ6AvXZ).
Registered report identifier: RR1-10.2196/10826.
Keywords: bupropion; genetics; internet; personality traits; smoking cessation; tobacco; varenicline.
©Laurie Zawertailo, Tara Mansoursadeghi-Gilan, Helena Zhang, Sarwar Hussain, Bernard Le Foll, Peter Selby. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 18.10.2018.
Conflict of interest statement
Conflicts of Interest: Over the last 5 years, PS has received grants from Pfizer Inc, Shoppers Drug Mart, Bhasin Consulting Fund Inc, and Patient-Centered Outcomes Research Institute. BLF receives support from Pfizer Global Research Awards in Nicotine Dependence Award Program.
Figures
References
-
- Health Canada Canadian Tobacco Use Monitoring Survey (CTUMS) 2012 March. 2013. p. 31. https://www.canada.ca/en/health-canada/services/publications/healthy-liv....
-
- Rehm JB, Brochu S, Fischer B, Gnam W, Patra J. The costs of substance abuse in Canada. Ottawa: Canadian Centre on Substance Abuse. 2006;Ottawa
-
- Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000 Aug 05;321(7257):355–8. http://europepmc.org/abstract/MED/10926597 - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical