Drosophila Ror is a nervous system-specific co-receptor for Wnt ligands
- PMID: 30341100
- PMCID: PMC6262871
- DOI: 10.1242/bio.033001
Drosophila Ror is a nervous system-specific co-receptor for Wnt ligands
Abstract
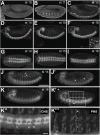
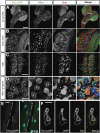
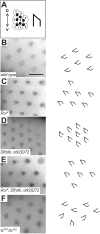
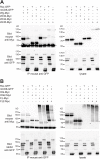
Wnt ligands are secreted glycoproteins that control many developmental processes and are crucial for homeostasis of numerous tissues in the adult organism. Signal transduction of Wnts involves the binding of Wnts to receptor complexes at the surface of target cells. These receptor complexes are commonly formed between a member of the Frizzled family of seven-pass transmembrane proteins and a co-receptor, which is usually a single-pass transmembrane protein. Among these co-receptors are several with structural homology to receptor tyrosine kinases, including Ror, PTK7, Ryk and MUSK. In vertebrates, Ror-2 and PTK7 are important regulators of planar cell polarity (PCP). By contrast, PCP phenotypes were not reported for mutations in off-track (otk) and off-track2 (otk2), encoding the Drosophila orthologs of PTK7. Here we show that Drosophila Ror is expressed in the nervous system and localizes to the plasma membrane of perikarya and neurites. A null allele of Ror is homozygous viable and fertile, does not display PCP phenotypes and interacts genetically with mutations in otk and otk2 We show that Ror binds specifically to Wingless (Wg), Wnt4 and Wnt5 and also to Frizzled2 (Fz2) and Otk. Our findings establish Drosophila Ror as a Wnt co-receptor expressed in the nervous system.
Keywords: Nervous system development; Planar cell polarity; Ror; Wnt signaling.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



Similar articles
-
ROR and RYK extracellular region structures suggest that receptor tyrosine kinases have distinct WNT-recognition modes.Cell Rep. 2021 Oct 19;37(3):109834. doi: 10.1016/j.celrep.2021.109834. Cell Rep. 2021. PMID: 34686333 Free PMC article.
-
The PTK7-related transmembrane proteins off-track and off-track 2 are co-receptors for Drosophila Wnt2 required for male fertility.PLoS Genet. 2014 Jul 10;10(7):e1004443. doi: 10.1371/journal.pgen.1004443. eCollection 2014 Jul. PLoS Genet. 2014. PMID: 25010066 Free PMC article.
-
PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling.EMBO J. 2011 Jul 19;30(18):3729-40. doi: 10.1038/emboj.2011.236. EMBO J. 2011. PMID: 21772251 Free PMC article.
-
The biochemistry, signalling and disease relevance of RYK and other WNT-binding receptor tyrosine kinases.Growth Factors. 2018 Apr;36(1-2):15-40. doi: 10.1080/08977194.2018.1472089. Epub 2018 May 28. Growth Factors. 2018. PMID: 29806777 Review.
-
ROR-Family Receptor Tyrosine Kinases.Curr Top Dev Biol. 2017;123:105-142. doi: 10.1016/bs.ctdb.2016.09.003. Epub 2016 Oct 31. Curr Top Dev Biol. 2017. PMID: 28236965 Review.
Cited by
-
Cephalic ganglia transcriptomics of the American cockroach Periplaneta americana (Blattodea: Blattidae).J Insect Sci. 2024 Nov 1;24(6):12. doi: 10.1093/jisesa/ieae113. J Insect Sci. 2024. PMID: 39688382 Free PMC article.
-
Inter-organ Wingless/Ror/Akt signaling regulates nutrient-dependent hyperarborization of somatosensory neurons.Elife. 2023 Jan 17;12:e79461. doi: 10.7554/eLife.79461. Elife. 2023. PMID: 36647607 Free PMC article.
-
The receptor tyrosine kinase Ror is required for dendrite regeneration in Drosophila neurons.PLoS Biol. 2020 Mar 12;18(3):e3000657. doi: 10.1371/journal.pbio.3000657. eCollection 2020 Mar. PLoS Biol. 2020. PMID: 32163406 Free PMC article.
-
Frizzled2 receives WntA signaling during butterfly wing pattern formation.Development. 2023 Sep 15;150(18):dev201868. doi: 10.1242/dev.201868. Epub 2023 Sep 28. Development. 2023. PMID: 37602496 Free PMC article.
-
ROR and RYK extracellular region structures suggest that receptor tyrosine kinases have distinct WNT-recognition modes.Cell Rep. 2021 Oct 19;37(3):109834. doi: 10.1016/j.celrep.2021.109834. Cell Rep. 2021. PMID: 34686333 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

