Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia
- PMID: 30341132
- PMCID: PMC6196842
- DOI: 10.1136/bmjopen-2018-023263
Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia
Abstract
Objective: To actively solicit adverse events experienced in the days following immunisation with quadrivalent inactivated influenza vaccine using Australia's near real-time, participant-based vaccine safety surveillance system, AusVaxSafety.
Design and setting: Observational cohort study conducted in 194 sentinel surveillance immunisation sites (primary care, hospital and community-based clinics) across Australia.
Participants: Individuals aged ≥6 months who received a routine seasonal influenza vaccine at a participating site (n=102 911) and responded to a survey (via short message service or email) sent 3 days after vaccination about adverse events experienced (n=73 892; 71.8%).
Main outcome measure: Near real-time and cumulative participant-reported rates of any adverse event, fever or medical attendance experienced within 3 days after vaccination overall, by brand, age, pregnancy status and concomitant vaccine receipt.
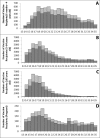
Results: Participant median age was 57 years (range: 6 months to 102 years); 58.1% (n=42 869) were female and 2.7% (n=2018) were pregnant. Near real-time fast initial response cumulative summation and Bayesian analyses of weekly event rates did not demonstrate a safety signal. Children aged 6 months to 4 years had higher event rates (522/6180; 8.4%) compared with older ages; participants aged ≥65 years reported fewer events (1695/28 154; 6.0%). There were no clinically significant differences in safety between brands, by age group or overall. Cumulative data analysis demonstrated that concomitant vaccination was associated with increased rates of fever (2.1% vs 0.8%) and medical attendance (0.8% vs 0.4%), although all rates were low and did not exceed expected levels.
Conclusions: Novel, postmarketing AusVaxSafety surveillance demonstrated comparable and expected safety outcomes for the 2017 quadrivalent inactivated influenza vaccine brands used in Australia. These near real-time, participant-reported data are expected to encourage confidence in vaccine safety and promote uptake.
Keywords: active surveillance; epidemiology; vaccine safety.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors are either located at organisations that hold the AusVaxSafety contract from the Australian Government Department of Health or are subcontract holders.
Figures
Similar articles
-
Challenges in implementing yearly enhanced safety surveillance of influenza vaccination in Europe: lessons learned and future perspectives.Hum Vaccin Immunother. 2019;15(11):2624-2636. doi: 10.1080/21645515.2019.1608745. Epub 2019 May 22. Hum Vaccin Immunother. 2019. PMID: 31116631 Free PMC article. Review.
-
Active SMS-based influenza vaccine safety surveillance in Australian children.Vaccine. 2017 Dec 18;35(51):7101-7106. doi: 10.1016/j.vaccine.2017.10.091. Epub 2017 Nov 8. Vaccine. 2017. PMID: 29128379
-
Comparative Postmarket Safety Profile of Adjuvanted and High-Dose Influenza Vaccines in Individuals 65 Years or Older.JAMA Netw Open. 2020 May 1;3(5):e204079. doi: 10.1001/jamanetworkopen.2020.4079. JAMA Netw Open. 2020. PMID: 32369177 Free PMC article.
-
Real-time safety surveillance of seasonal influenza vaccines in children, Australia, 2015.Euro Surveill. 2015;20(43). doi: 10.2807/1560-7917.ES.2015.20.43.30050. Euro Surveill. 2015. PMID: 26536867
-
Seasonal influenza vaccines.Curr Top Microbiol Immunol. 2009;333:43-82. doi: 10.1007/978-3-540-92165-3_3. Curr Top Microbiol Immunol. 2009. PMID: 19768400 Review.
Cited by
-
Challenges in implementing yearly enhanced safety surveillance of influenza vaccination in Europe: lessons learned and future perspectives.Hum Vaccin Immunother. 2019;15(11):2624-2636. doi: 10.1080/21645515.2019.1608745. Epub 2019 May 22. Hum Vaccin Immunother. 2019. PMID: 31116631 Free PMC article. Review.
-
The short term safety of COVID-19 vaccines in Australia: AusVaxSafety active surveillance, February - August 2021.Med J Aust. 2022 Aug 15;217(4):195-202. doi: 10.5694/mja2.51619. Epub 2022 Jul 4. Med J Aust. 2022. PMID: 35781813 Free PMC article.
-
A Web-Based Tool to Report Adverse Drug Reactions by Community Pharmacists in Australia: Usability Testing Study.JMIR Form Res. 2023 Sep 29;7:e48976. doi: 10.2196/48976. JMIR Form Res. 2023. PMID: 37773620 Free PMC article.
-
Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia.Prev Med Rep. 2021 Oct 11;24:101595. doi: 10.1016/j.pmedr.2021.101595. eCollection 2021 Dec. Prev Med Rep. 2021. PMID: 34976653 Free PMC article.
-
Exposure to quadrivalent influenza vaccine during pregnancy: Results from a global pregnancy registry.Influenza Other Respir Viruses. 2022 Jan;16(1):90-100. doi: 10.1111/irv.12897. Epub 2021 Sep 14. Influenza Other Respir Viruses. 2022. PMID: 34520127 Free PMC article.
References
-
- Palache A, Abelin A, Hollingsworth R, et al. . Survey of distribution of seasonal influenza vaccine doses in 201 countries (2004-2015): The 2003 World Health Assembly resolution on seasonal influenza vaccination coverage and the 2009 influenza pandemic have had very little impact on improving influenza control and pandemic preparedness. Vaccine 2017;35:4681–6. 10.1016/j.vaccine.2017.07.053 - DOI - PubMed
-
- Agency EM. Guideline on influenza vaccines non-clinical and clinical module. London, UK: European Medicines Agency, 2016.
-
- The Royal Children’s Hospital Melbourne. Vaccination: perspectives of Australian parents. The Royal Children’s Hospital National Child Health Poll, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases