Advance Care Planning and HIV Symptoms in Adolescence
- PMID: 30341154
- PMCID: PMC6317555
- DOI: 10.1542/peds.2017-3869
Advance Care Planning and HIV Symptoms in Adolescence
Abstract
Objectives: To determine the effect of family-centered pediatric advance care planning (FACE pACP) on HIV-specific symptoms.
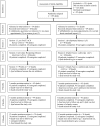
Methods: In this single-blinded, randomized controlled trial conducted at 6 US hospital-based HIV clinics, 105 adolescent-family dyads, randomly assigned from July 2011 to June 2014, received 3 weekly sessions in either the FACE pACP arm ([1] pediatric advance care planning survey, [2] Respecting Choices interview, and [3] 5 Wishes directive) or the control arm ([1] developmental history, [2] safety tips, and [3] nutrition and exercise tips). The General Health Assessment for Children measured patient-reported HIV-specific symptoms. Latent class analyses clustered individual patients based on symptom patterns. Path analysis examined the mediating role of dyadic treatment congruence with respect to the intervention effect on symptom patterns.
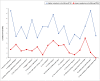
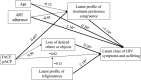
Results: Patients were a mean age of 17.8 years old, 54% male, and 93% African American. Latent class analysis identified 2 latent HIV-symptom classes at 12 months: higher symptoms and suffering (27%) and lower symptoms and suffering (73%). FACE pACP had a positive effect on dyadic treatment congruence (β = .65; 95% CI: 0.04 to 1.28), and higher treatment congruence had a negative effect on symptoms and suffering (β = -1.14; 95% CI: -2.55 to -0.24). Therefore, FACE pACP decreased the likelihood of symptoms and suffering through better dyadic treatment congruence (β = -.69; 95% CI: -2.14 to -0.006). Higher religiousness (β = 2.19; 95% CI: 0.22 to 4.70) predicted symptoms and suffering.
Conclusions: FACE pACP increased and maintained agreement about goals of care longitudinally, which lowered adolescents' physical symptoms and suffering, suggesting that early pACP is worthwhile.
Copyright © 2018 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Lyon developed and adapted the Lyon Advance Care Planning Survey adolescent and surrogate versions used in session 1 of the 3-session intervention. Ms Briggs developed and adapted the statement of treatment preferences used to obtain study results on treatment agreement between the adolescent and family; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures

References
-
- Neilan AM, Karalius B, Patel K, et al. ; Pediatric HIV/AIDS Cohort Study; International Maternal Adolescent and Pediatric AIDS Clinical Trials Network . Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally human immunodeficiency virus-infected youth. JAMA Pediatr. 2017;171(5):450–460 - PMC - PubMed
-
- Centers for Disease Control and Prevention HIV among youth. 2018. Available at: https://www.cdc.gov/hiv/group/age/youth/. Accessed June 5, 2018
-
- UNAIDS All in to #EndAdolescentAIDS. 2015. Available at: www.unaids.org/sites/default/files/media_asset/20150217_ALL_IN_brochure.pdf. Accessed June 5, 2018
-
- Rietjens JAC, Sudore RL, Connolly M, et al. ; European Association for Palliative Care . Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–e551 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

