ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-β-hydroxylase
- PMID: 30341172
- PMCID: PMC6311498
- DOI: 10.1074/jbc.RA118.004889
ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-β-hydroxylase
Abstract
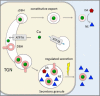
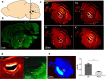
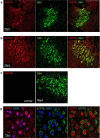
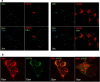
The copper (Cu) transporters ATPase copper-transporting alpha (ATP7A) and ATPase copper-transporting beta (ATP7B) are essential for the normal function of the mammalian central nervous system. Inactivation of ATP7A or ATP7B causes the severe neurological disorders, Menkes disease and Wilson disease, respectively. In both diseases, Cu imbalance is associated with abnormal levels of the catecholamine-type neurotransmitters dopamine and norepinephrine. Dopamine is converted to norepinephrine by dopamine-β-hydroxylase (DBH), which acquires its essential Cu cofactor from ATP7A. However, the role of ATP7B in catecholamine homeostasis is unclear. Here, using immunostaining of mouse brain sections and cultured cells, we show that DBH-containing neurons express both ATP7A and ATP7B. The two transporters are located in distinct cellular compartments and oppositely regulate the export of soluble DBH from cultured neuronal cells under resting conditions. Down-regulation of ATP7A, overexpression of ATP7B, and pharmacological Cu depletion increased DBH retention in cells. In contrast, ATP7B inactivation elevated extracellular DBH. Proteolytic processing and the specific activity of exported DBH were not affected by changes in ATP7B levels. These results establish distinct regulatory roles for ATP7A and ATP7B in neuronal cells and explain, in part, the lack of functional compensation between these two transporters in human disorders of Cu imbalance.
Keywords: ATP7A; ATP7B; Menkes disease; SH-SY5Y cells; Wilson's disease; cellular regulation; constitutive secretion; copper transport; dopamine-β-hydroxylase; intracellular trafficking; locus coeruleus; noradrenergic; vesicles.
© 2018 Schmidt et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum.J Biol Chem. 2005 Mar 11;280(10):9640-5. doi: 10.1074/jbc.M413840200. Epub 2005 Jan 5. J Biol Chem. 2005. PMID: 15634671
-
A mutation in the ATP7B copper transporter causes reduced dopamine beta-hydroxylase and norepinephrine in mouse adrenal.Neurochem Res. 2003 Jun;28(6):867-73. doi: 10.1023/a:1023219308890. Neurochem Res. 2003. PMID: 12718440
-
Atp7b-dependent choroid plexus dysfunction causes transient copper deficit and metabolic changes in the developing mouse brain.PLoS Genet. 2023 Jan 10;19(1):e1010558. doi: 10.1371/journal.pgen.1010558. eCollection 2023 Jan. PLoS Genet. 2023. PMID: 36626371 Free PMC article.
-
Function and regulation of human copper-transporting ATPases.Physiol Rev. 2007 Jul;87(3):1011-46. doi: 10.1152/physrev.00004.2006. Physiol Rev. 2007. PMID: 17615395 Review.
-
Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins.J Bioenerg Biomembr. 2007 Dec;39(5-6):403-7. doi: 10.1007/s10863-007-9101-2. J Bioenerg Biomembr. 2007. PMID: 18000748 Review.
Cited by
-
Subnormal Serum Liver Enzyme Levels: A Review of Pathophysiology and Clinical Significance.J Clin Transl Hepatol. 2024 Apr 28;12(4):428-435. doi: 10.14218/JCTH.2023.00446. Epub 2024 Mar 18. J Clin Transl Hepatol. 2024. PMID: 38638374 Free PMC article. Review.
-
The Oncopig as an Emerging Model to Investigate Copper Regulation in Cancer.Int J Mol Sci. 2022 Nov 13;23(22):14012. doi: 10.3390/ijms232214012. Int J Mol Sci. 2022. PMID: 36430490 Free PMC article. Review.
-
Transmembrane Cu(I) P-type ATPase pumps are electrogenic uniporters.Dalton Trans. 2020 Nov 25;49(45):16082-16094. doi: 10.1039/d0dt01380c. Dalton Trans. 2020. PMID: 32469032 Free PMC article.
-
Copper homeostasis and pregnancy complications: a comprehensive review.J Assist Reprod Genet. 2025 Mar;42(3):707-720. doi: 10.1007/s10815-024-03375-4. Epub 2025 Jan 10. J Assist Reprod Genet. 2025. PMID: 39792348 Review.
-
Elevated Expression of PDZD11 Is Associated With Poor Prognosis and Immune Infiltrates in Hepatocellular Carcinoma.Front Genet. 2021 May 21;12:669928. doi: 10.3389/fgene.2021.669928. eCollection 2021. Front Genet. 2021. PMID: 34093661 Free PMC article.
References
-
- Dodani S. C., Firl A., Chan J., Nam C. I., Aron A. T., Onak C. S., Ramos-Torres K. M., Paek J., Webster C. M., Feller M. B., and Chang C. J. (2014) Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl. Acad. Sci. U.S.A. 111, 16280–16285 10.1073/pnas.1409796111 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

