MicroRNA-495-3p inhibits multidrug resistance by modulating autophagy through GRP78/mTOR axis in gastric cancer
- PMID: 30341283
- PMCID: PMC6195618
- DOI: 10.1038/s41419-018-0950-x
MicroRNA-495-3p inhibits multidrug resistance by modulating autophagy through GRP78/mTOR axis in gastric cancer
Abstract
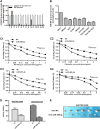
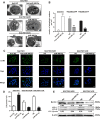
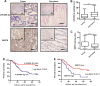
Multidrug resistance (MDR) accounts for poor prognosis in gastric cancer (GC). MicroRNAs (miRNAs) are critical regulators of MDR via modulation of the target genes. The present study revealed that miR-495-3p could act via a target gene, GRP78, to regulate the process of autophagy and inhibit MDR. Based on the in vitro and in vivo gain-of-function or loss-of-function experiments, overexpression of miR-495-3p was sufficient to reverse the MDR to four chemotherapeutics in vitro and inhibit the tumor growth in vivo. Moreover, GRP78 was positively associated with the occurrence of autophagy. Thus, reducing the expression of GRP78 by siRNA resulted in autophagy-suppressive activity similar to that of miR-495-3p on mammalian target of rapamycin (mTOR) and its substrates activation and autophagy inhibition, while restoring GRP78 attenuated the anti-autophagy effects caused by miR-495-3p. Clinically, either miR-495-3p downregulation or GRP78 upregulation was associated with malignant phenotypes in patients with GC. In conclusion, these findings demonstrate that miR-495-3p is an important regulator of autophagy balance and MDR by modulating the GRP78/mTOR axis. In addition, miR-495-3p and GRP78 could be used as prognostic factors for overall survival in GC, which implicates miR-495-3p as a therapeutic target in cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Liu Y, Peng H, Zhang JT. Expression profiling of ABC transporters in a drug-resistant breast cancer cell line using AmpArray. Mol. Pharmacol. 2005;68:430–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

