Common mechanism of transcription termination at coding and noncoding RNA genes in fission yeast
- PMID: 30341288
- PMCID: PMC6195540
- DOI: 10.1038/s41467-018-06546-x
Common mechanism of transcription termination at coding and noncoding RNA genes in fission yeast
Abstract
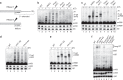
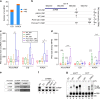
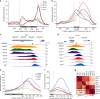
Termination of RNA polymerase II (RNAPII) transcription is a fundamental step of gene expression that is critical for determining the borders between genes. In budding yeast, termination at protein-coding genes is initiated by the cleavage/polyadenylation machinery, whereas termination of most noncoding RNA (ncRNA) genes occurs via the Nrd1-Nab3-Sen1 (NNS) pathway. Here, we find that NNS-like transcription termination is not conserved in fission yeast. Rather, genome-wide analyses show global recruitment of mRNA 3' end processing factors at the end of ncRNA genes, including snoRNAs and snRNAs, and that this recruitment coincides with high levels of Ser2 and Tyr1 phosphorylation on the RNAPII C-terminal domain. We also find that termination of mRNA and ncRNA transcription requires the conserved Ysh1/CPSF-73 and Dhp1/XRN2 nucleases, supporting widespread cleavage-dependent transcription termination in fission yeast. Our findings thus reveal that a common mode of transcription termination can produce functionally and structurally distinct types of polyadenylated and non-polyadenylated RNAs.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
RNA Polymerase II CTD Tyrosine 1 Is Required for Efficient Termination by the Nrd1-Nab3-Sen1 Pathway.Mol Cell. 2019 Feb 21;73(4):655-669.e7. doi: 10.1016/j.molcel.2018.12.002. Epub 2019 Jan 10. Mol Cell. 2019. PMID: 30639244
-
The exosome component Rrp6 is required for RNA polymerase II termination at specific targets of the Nrd1-Nab3 pathway.PLoS Genet. 2015 Feb 13;11(2):e1004999. doi: 10.1371/journal.pgen.1004999. eCollection 2015. PLoS Genet. 2015. PMID: 25680078 Free PMC article.
-
The role of Ctk1 kinase in termination of small non-coding RNAs.PLoS One. 2013 Dec 4;8(12):e80495. doi: 10.1371/journal.pone.0080495. eCollection 2013. PLoS One. 2013. PMID: 24324601 Free PMC article.
-
The Nrd1-Nab3-Sen1 transcription termination complex from a structural perspective.Biochem Soc Trans. 2023 Jun 28;51(3):1257-1269. doi: 10.1042/BST20221418. Biochem Soc Trans. 2023. PMID: 37222282 Free PMC article. Review.
-
Transcription termination and the control of the transcriptome: why, where and how to stop.Nat Rev Mol Cell Biol. 2015 Mar;16(3):190-202. doi: 10.1038/nrm3943. Epub 2015 Feb 4. Nat Rev Mol Cell Biol. 2015. PMID: 25650800 Review.
Cited by
-
Transcriptional co-activators: emerging roles in signaling pathways and potential therapeutic targets for diseases.Signal Transduct Target Ther. 2023 Nov 13;8(1):427. doi: 10.1038/s41392-023-01651-w. Signal Transduct Target Ther. 2023. PMID: 37953273 Free PMC article. Review.
-
Transcriptional regulation of FACT involves Coordination of chromatin accessibility and CTCF binding.J Biol Chem. 2024 Jan;300(1):105538. doi: 10.1016/j.jbc.2023.105538. Epub 2023 Dec 10. J Biol Chem. 2024. PMID: 38072046 Free PMC article.
-
Epigenome Mapping in Quiescent Cells Reveals a Key Role for H3K4me3 in Regulation of RNA Polymerase II Activity.Epigenomes. 2024 Oct 22;8(4):39. doi: 10.3390/epigenomes8040039. Epigenomes. 2024. PMID: 39449363 Free PMC article.
-
Nrd1p identifies aberrant and natural exosomal target messages during the nuclear mRNA surveillance in Saccharomyces cerevisiae.Nucleic Acids Res. 2021 Nov 18;49(20):11512-11536. doi: 10.1093/nar/gkab930. Nucleic Acids Res. 2021. PMID: 34664673 Free PMC article.
-
Post-transcriptional polyadenylation site cleavage maintains 3'-end processing upon DNA damage.EMBO J. 2023 Apr 3;42(7):e112358. doi: 10.15252/embj.2022112358. Epub 2023 Feb 10. EMBO J. 2023. PMID: 36762421 Free PMC article.
References
-
- Baejen Carlo, Andreani Jessica, Torkler Phillipp, Battaglia Sofia, Schwalb Bjoern, Lidschreiber Michael, Maier Kerstin C., Boltendahl Andrea, Rus Petra, Esslinger Stephanie, Söding Johannes, Cramer Patrick. Genome-wide Analysis of RNA Polymerase II Termination at Protein-Coding Genes. Molecular Cell. 2017;66(1):38-49.e6. doi: 10.1016/j.molcel.2017.02.009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

