Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens
- PMID: 30341305
- PMCID: PMC6195583
- DOI: 10.1038/s41467-018-06849-z
Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens
Abstract
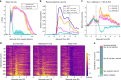
The ventral striatopallidal system, a basal ganglia network thought to convert limbic information into behavioral action, includes the nucleus accumbens (NAc) and the ventral pallidum (VP), typically described as a major output of NAc. Here, to investigate how reward-related information is transformed across this circuit, we measure the activity of neurons in NAc and VP when rats receive two highly palatable but differentially preferred rewards, allowing us to track the reward-specific information contained within the neural activity of each region. In VP, we find a prominent preference-related signal that flexibly reports the relative value of reward outcomes across multiple conditions. This reward-specific firing in VP is present in a greater proportion of the population and arises sooner following reward delivery than in NAc. Our findings establish VP as a preeminent value signaler and challenge the existing model of information flow in the ventral basal ganglia.
Conflict of interest statement
The authors declare no competing interests.
Figures
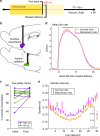
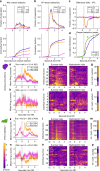

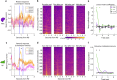
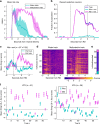
References
Publication types
MeSH terms
Grants and funding
- K99 AA025384/AA/NIAAA NIH HHS/United States
- K99AA025384/U.S. Department of Health & Human Services | NIH | National Institute on Alcohol Abuse and Alcoholism (NIAAA)/International
- R01 DA035943/DA/NIDA NIH HHS/United States
- R01DA035943/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- T32 NS091018/NS/NINDS NIH HHS/United States
- R01 AA026306/AA/NIAAA NIH HHS/United States
- 5T32NS91018-17/U.S. Department of Health & Human Services | National Institutes of Health (NIH)/International
- Young Investigator Award/National Alliance for Research on Schizophrenia and Depression (NARSAD)/International
- DGE-1746891/National Science Foundation (NSF)/International
LinkOut - more resources
Full Text Sources

