SUMOgo: Prediction of sumoylation sites on lysines by motif screening models and the effects of various post-translational modifications
- PMID: 30341374
- PMCID: PMC6195521
- DOI: 10.1038/s41598-018-33951-5
SUMOgo: Prediction of sumoylation sites on lysines by motif screening models and the effects of various post-translational modifications
Abstract
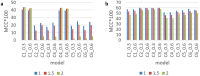
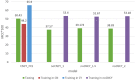
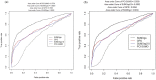
Most modern tools used to predict sites of small ubiquitin-like modifier (SUMO) binding (referred to as SUMOylation) use algorithms, chemical features of the protein, and consensus motifs. However, these tools rarely consider the influence of post-translational modification (PTM) information for other sites within the same protein on the accuracy of prediction results. This study applied the Random Forest machine learning method, as well as motif screening models and a feature selection combination mechanism, to develop a SUMOylation prediction system, referred to as SUMOgo. With regard to prediction method, PTM sites were coded as new functional features in addition to structural features, such as sequence-based binary coding, encoded chemical features of proteins, and encoded secondary structure information that is important for PTM. Twenty cycles of prediction were conducted with a 1:1 combination of positive test data and random negative data. Matthew's correlation coefficient of SUMOgo reached 0.511, which is higher than that of current commonly used tools. This study further verified the important role of PTM in SUMOgo and includes a case study on CREB binding protein (CREBBP). The website for the final tool is http://predictor.nchu.edu.tw/SUMOgo .
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Protein sumoylation sites prediction based on two-stage feature selection.Mol Divers. 2010 Feb;14(1):81-6. doi: 10.1007/s11030-009-9149-5. Epub 2009 May 27. Mol Divers. 2010. PMID: 19472067
-
SUMO-Forest: A Cascade Forest based method for the prediction of SUMOylation sites on imbalanced data.Gene. 2020 May 30;741:144536. doi: 10.1016/j.gene.2020.144536. Epub 2020 Mar 8. Gene. 2020. PMID: 32160959 No abstract available.
-
Predicting protein sumoylation sites from sequence features.Amino Acids. 2012 Jul;43(1):447-55. doi: 10.1007/s00726-011-1100-2. Epub 2011 Oct 7. Amino Acids. 2012. PMID: 21986959
-
Recent Development of Machine Learning Methods in Sumoylation Sites Prediction.Curr Med Chem. 2022;29(5):894-907. doi: 10.2174/0929867328666210915112030. Curr Med Chem. 2022. PMID: 34525906 Review.
-
Large-scale comparative assessment of computational predictors for lysine post-translational modification sites.Brief Bioinform. 2019 Nov 27;20(6):2267-2290. doi: 10.1093/bib/bby089. Brief Bioinform. 2019. PMID: 30285084 Free PMC article. Review.
Cited by
-
Humanization reveals pervasive incompatibility of yeast and human kinetochore components.G3 (Bethesda). 2023 Dec 29;14(1):jkad260. doi: 10.1093/g3journal/jkad260. G3 (Bethesda). 2023. PMID: 37962556 Free PMC article.
-
Computational Analysis Indicates That PARP1 Acts as a Histone Deacetylases Interactor Sharing Common Lysine Residues for Acetylation, Ubiquitination, and SUMOylation in Alzheimer's and Parkinson's Disease.ACS Omega. 2021 Feb 19;6(8):5739-5753. doi: 10.1021/acsomega.0c06168. eCollection 2021 Mar 2. ACS Omega. 2021. PMID: 33681613 Free PMC article.
-
Sitetack: a deep learning model that improves PTM prediction by using known PTMs.Bioinformatics. 2024 Nov 1;40(11):btae602. doi: 10.1093/bioinformatics/btae602. Bioinformatics. 2024. PMID: 39388212 Free PMC article.
-
iSUMOK-PseAAC: prediction of lysine sumoylation sites using statistical moments and Chou's PseAAC.PeerJ. 2021 Aug 4;9:e11581. doi: 10.7717/peerj.11581. eCollection 2021. PeerJ. 2021. PMID: 34430072 Free PMC article.
-
Thirty years of molecular dynamics simulations on posttranslational modifications of proteins.Phys Chem Chem Phys. 2022 Nov 9;24(43):26371-26397. doi: 10.1039/d2cp02883b. Phys Chem Chem Phys. 2022. PMID: 36285789 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

