Relative positioning of Kv11.1 (hERG) K+ channel cytoplasmic domain-located fluorescent tags toward the plasma membrane
- PMID: 30341381
- PMCID: PMC6195548
- DOI: 10.1038/s41598-018-33492-x
Relative positioning of Kv11.1 (hERG) K+ channel cytoplasmic domain-located fluorescent tags toward the plasma membrane
Abstract
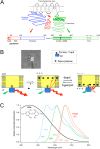
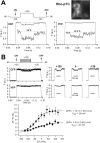
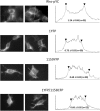
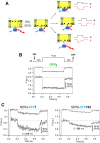
Recent cryo-EM data have provided a view of the KCNH potassium channels molecular structures. However, some details about the cytoplasmic domains organization and specially their rearrangements associated to channel functionality are still lacking. Here we used the voltage-dependent dipicrylamine (DPA)-induced quench of fluorescent proteins (FPS) linked to different positions at the cytoplasmic domains of KCNH2 (hERG) to gain some insights about the coarse structure of these channel parts. Fast voltage-clamp fluorometry with HEK293 cells expressing membrane-anchored FPs under conditions in which only the plasma membrane potential is modified, demonstrated DPA voltage-dependent translocation and subsequent FRET-triggered FP quenching. Our data demonstrate for the first time that the distance between an amino-terminal FP tag and the intracellular plasma membrane surface is shorter than that between the membrane and a C-terminally-located tag. The distances varied when the FPs were attached to other positions along the channel cytoplasmic domains. In some cases, we also detected slower fluorometric responses following the fast voltage-dependent dye translocation, indicating subsequent label movements orthogonal to the plasma membrane. This finding suggests the existence of additional conformational rearrangements in the hERG cytoplasmic domains, although their association with specific aspects of channel operation remains to be established.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Gating mechanism of Kv11.1 (hERG) K+ channels without covalent connection between voltage sensor and pore domains.Pflugers Arch. 2018 Mar;470(3):517-536. doi: 10.1007/s00424-017-2093-9. Epub 2017 Dec 21. Pflugers Arch. 2018. PMID: 29270671 Free PMC article.
-
Cryo-EM Structure of the Open Human Ether-à-go-go-Related K+ Channel hERG.Cell. 2017 Apr 20;169(3):422-430.e10. doi: 10.1016/j.cell.2017.03.048. Cell. 2017. PMID: 28431243 Free PMC article.
-
Regulation of Kv11.1 potassium channel C-terminal isoform expression by the RNA-binding proteins HuR and HuD.J Biol Chem. 2018 Dec 21;293(51):19624-19632. doi: 10.1074/jbc.RA118.003720. Epub 2018 Oct 29. J Biol Chem. 2018. PMID: 30377250 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Towards a Structural View of Drug Binding to hERG K+ Channels.Trends Pharmacol Sci. 2017 Oct;38(10):899-907. doi: 10.1016/j.tips.2017.06.004. Epub 2017 Jul 12. Trends Pharmacol Sci. 2017. PMID: 28711156 Free PMC article. Review.
Cited by
-
Refinement of a cryo-EM structure of hERG: Bridging structure and function.Biophys J. 2021 Feb 16;120(4):738-748. doi: 10.1016/j.bpj.2021.01.011. Epub 2021 Jan 19. Biophys J. 2021. PMID: 33476597 Free PMC article.
-
New Structures and Gating of Voltage-Dependent Potassium (Kv) Channels and Their Relatives: A Multi-Domain and Dynamic Question.Int J Mol Sci. 2019 Jan 10;20(2):248. doi: 10.3390/ijms20020248. Int J Mol Sci. 2019. PMID: 30634573 Free PMC article. Review.
-
Structures Illuminate Cardiac Ion Channel Functions in Health and in Long QT Syndrome.Front Pharmacol. 2020 May 4;11:550. doi: 10.3389/fphar.2020.00550. eCollection 2020. Front Pharmacol. 2020. PMID: 32431610 Free PMC article. Review.
-
Topography and motion of acid-sensing ion channel intracellular domains.Elife. 2021 Jul 22;10:e68955. doi: 10.7554/eLife.68955. Elife. 2021. PMID: 34292153 Free PMC article.
-
Conformation-sensitive antibody reveals an altered cytosolic PAS/CNBh assembly during hERG channel gating.Proc Natl Acad Sci U S A. 2021 Nov 2;118(44):e2108796118. doi: 10.1073/pnas.2108796118. Proc Natl Acad Sci U S A. 2021. PMID: 34716268 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

