Pyroptosis versus necroptosis: similarities, differences, and crosstalk
- PMID: 30341423
- PMCID: PMC6294779
- DOI: 10.1038/s41418-018-0212-6
Pyroptosis versus necroptosis: similarities, differences, and crosstalk
Abstract
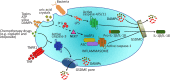
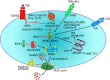
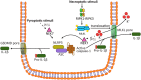
Pyroptosis and necroptosis represent two pathways of genetically encoded necrotic cell death. Although these cell death programmes can protect the host against microbial pathogens, their dysregulation has been implicated in a variety of autoimmune and auto-inflammatory conditions. The disease-promoting potential of necroptosis and pyroptosis is likely a consequence of their ability to induce a lytic cell death. This cell suicide mechanism, distinct from apoptosis, allows the release of immunogenic cellular content, including damage-associated molecular patterns (DAMPs), and inflammatory cytokines such as interleukin-1β (IL-1β), to trigger inflammation. In this Review, we discuss recent discoveries that have advanced our understanding on the primary functions of pyroptosis and necroptosis, including evidence for the specific cytokines and DAMPs responsible for driving inflammation. We compare the similar and unique aspects of pyroptotic- and necroptotic-induced membrane damage, and explore how these may functionally impact distinct intracellular organelles and signalling pathways. We also examine studies highlighting the crosstalk that can occur between necroptosis and pyroptosis signalling, and evidence supporting the physiological significance of this convergence. Ultimately, a better understanding of the similarities, unique aspects and crosstalk of pyroptosis and necroptosis will inform as to how these cell death pathways might be manipulated for therapeutic benefit.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Lockshin RA, Williams CM. Programmed cell death--I. Cytology of degeneration in the intersegmental muscles of the Pernyi silkmoth. J Insect Physiol. 1965;11:123–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

