Deficiency of the clock gene Bmal1 affects neural progenitor cell migration
- PMID: 30341743
- PMCID: PMC6373387
- DOI: 10.1007/s00429-018-1775-1
Deficiency of the clock gene Bmal1 affects neural progenitor cell migration
Abstract
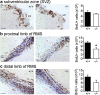
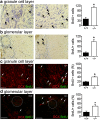
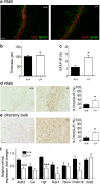
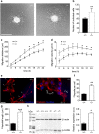
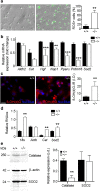
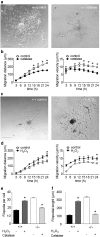
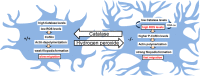
We demonstrate the impact of a disrupted molecular clock in Bmal1-deficient (Bmal1-/-) mice on migration of neural progenitor cells (NPCs). Proliferation of NPCs in rostral migratory stream (RMS) was reduced in Bmal1-/- mice, consistent with our earlier studies on adult neurogenesis in hippocampus. However, a significantly higher number of NPCs from Bmal1-/- mice reached the olfactory bulb as compared to wild-type littermates (Bmal1+/+ mice), indicating a higher migration velocity in Bmal1-/- mice. In isolated NPCs from Bmal1-/- mice, not only migration velocity and expression pattern of genes involved in detoxification of reactive oxygen species were affected, but also RNA oxidation of catalase was increased and catalase protein levels were decreased. Bmal1+/+ migration phenotype could be restored by treatment with catalase, while treatment of NPCs from Bmal1+/+ mice with hydrogen peroxide mimicked Bmal1-/- migration phenotype. Thus, we conclude that Bmal1 deficiency affects NPC migration as a consequence of dysregulated detoxification of reactive oxygen species.
Keywords: Bmal1; Catalase; Circadian; Clock genes; Cytoskeleton; Filopodia; Hydrogen peroxide; RNA oxidation; Rostral migratory stream; Subventricular zone; p-Cofilin.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Impact of Targeted Deletion of the Circadian Clock Gene Bmal1 in Excitatory Forebrain Neurons on Adult Neurogenesis and Olfactory Function.Int J Mol Sci. 2020 Feb 19;21(4):1394. doi: 10.3390/ijms21041394. Int J Mol Sci. 2020. PMID: 32092990 Free PMC article.
-
Premature aging of the hippocampal neurogenic niche in adult Bmal1-deficient mice.Aging (Albany NY). 2015 Jun;7(6):435-49. doi: 10.18632/aging.100764. Aging (Albany NY). 2015. PMID: 26142744 Free PMC article.
-
MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: validation of various MPIO labeling strategies.Neuroimage. 2010 Feb 1;49(3):2094-103. doi: 10.1016/j.neuroimage.2009.10.034. Epub 2009 Oct 19. Neuroimage. 2010. PMID: 19850132
-
Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
-
Transcriptional regulation of olfactory bulb neurogenesis.Anat Rec (Hoboken). 2013 Sep;296(9):1364-82. doi: 10.1002/ar.22733. Epub 2013 Jul 31. Anat Rec (Hoboken). 2013. PMID: 23904336 Review.
Cited by
-
Impact of Targeted Deletion of the Circadian Clock Gene Bmal1 in Excitatory Forebrain Neurons on Adult Neurogenesis and Olfactory Function.Int J Mol Sci. 2020 Feb 19;21(4):1394. doi: 10.3390/ijms21041394. Int J Mol Sci. 2020. PMID: 32092990 Free PMC article.
-
The Relevance of Circadian Clocks to Stem Cell Differentiation and Cancer Progression.NeuroSci. 2022 Mar 29;3(2):146-165. doi: 10.3390/neurosci3020012. eCollection 2022 Jun. NeuroSci. 2022. PMID: 39483369 Free PMC article. Review.
-
Adult Neurogenesis under Control of the Circadian System.Cells. 2022 Feb 22;11(5):764. doi: 10.3390/cells11050764. Cells. 2022. PMID: 35269386 Free PMC article. Review.
-
Blood pressure regulation through circadian variation: PRDM16 as a target in vascular smooth muscle cells.J Clin Invest. 2025 Feb 3;135(3):e188784. doi: 10.1172/JCI188784. J Clin Invest. 2025. PMID: 39895629 Free PMC article.
-
Zika Virus Infection Alters the Circadian Clock Expression in Human Neuronal Monolayer and Neurosphere Cultures.Cell Mol Neurobiol. 2023 Dec 23;44(1):10. doi: 10.1007/s10571-023-01445-2. Cell Mol Neurobiol. 2023. PMID: 38141078 Free PMC article.
References
-
- Baumann J, et al. Comparative human and rat “neurosphere assay” for developmental neurotoxicity testing. Curr Protoc Toxicol. 2014;59:12. - PubMed
-
- Baumann JDK, Barenys M, Giersiefer S, Goniwiecha J, Lein PJ, Fritsche E. Application of the neurosphere assay for DNT hazard assessment: challenges and limitations. In: Kang YJ, editor. Methods in pharmacology and toxicology. Berlin: Springer; 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

