Methods to identify the substrates of thiol-disulfide oxidoreductases
- PMID: 30341785
- PMCID: PMC6295885
- DOI: 10.1002/pro.3530
Methods to identify the substrates of thiol-disulfide oxidoreductases
Abstract
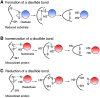
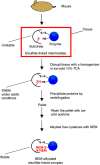
The formation of a disulfide bond is a critical step in the folding of numerous secretory and membrane proteins and catalyzed in vivo. A variety of mechanisms and protein structures have evolved to catalyze oxidative protein folding. Those enzymes that directly interact with a folding protein to accelerate its oxidative folding are mostly thiol-disulfide oxidoreductases that belong to the thioredoxin superfamily. The enzymes of this class often use a CXXC active-site motif embedded in their thioredoxin-like fold to promote formation, isomerization, and reduction of a disulfide bond in their target proteins. Over the past decade or so, an increasing number of substrates of the thiol-disulfide oxidoreductases that are present in the ER of mammalian cells have been discovered, revealing that the enzymes play unexpectedly diverse physiological functions. However, functions of some of these enzymes still remain unclear due to the lack of information on their substrates. Here, we review the methods used by researchers to identify the substrates of these enzymes and provide data that show the importance of using trichloroacetic acid in sample preparation for the substrate identification, hoping to aid future studies. We particularly focus on successful studies that have uncovered physiological substrates and functions of the enzymes in the periplasm of Gram-negative bacteria and the endoplasmic reticulum of mammalian cells. Similar approaches should be applicable to enzymes in other cellular compartments or in other organisms.
Keywords: disulfide bond; disulfide-linked enzyme-substrate complex; oxidative protein folding; protein disulfide isomerase; thiol-based redox regulation; thioredoxin superfamily member.
© 2018 The Protein Society.
Figures


Similar articles
-
The thioredoxin superfamily in oxidative protein folding.Antioxid Redox Signal. 2014 Jul 20;21(3):457-70. doi: 10.1089/ars.2014.5849. Epub 2014 Mar 6. Antioxid Redox Signal. 2014. PMID: 24483600 Review.
-
Cooperative Protein Folding by Two Protein Thiol Disulfide Oxidoreductases and 1 in Soybean.Plant Physiol. 2016 Feb;170(2):774-89. doi: 10.1104/pp.15.01781. Epub 2015 Dec 8. Plant Physiol. 2016. PMID: 26645455 Free PMC article.
-
Periplasmic protein thiol:disulfide oxidoreductases of Escherichia coli.FEMS Microbiol Rev. 2000 Jul;24(3):303-16. doi: 10.1111/j.1574-6976.2000.tb00544.x. FEMS Microbiol Rev. 2000. PMID: 10841975 Review.
-
Novel thioredoxin-related transmembrane protein TMX4 has reductase activity.J Biol Chem. 2010 Mar 5;285(10):7135-42. doi: 10.1074/jbc.M109.082545. Epub 2010 Jan 7. J Biol Chem. 2010. PMID: 20056998 Free PMC article.
-
Protein thiol modifications visualized in vivo.PLoS Biol. 2004 Nov;2(11):e333. doi: 10.1371/journal.pbio.0020333. Epub 2004 Oct 5. PLoS Biol. 2004. PMID: 15502869 Free PMC article.
Cited by
-
Stoichiometric Thiol Redox Proteomics for Quantifying Cellular Responses to Perturbations.Antioxidants (Basel). 2021 Mar 23;10(3):499. doi: 10.3390/antiox10030499. Antioxidants (Basel). 2021. PMID: 33807006 Free PMC article. Review.
-
Observing the nonvectorial yet cotranslational folding of a multidomain protein, LDL receptor, in the ER of mammalian cells.Proc Natl Acad Sci U S A. 2020 Jul 14;117(28):16401-16408. doi: 10.1073/pnas.2004606117. Epub 2020 Jun 29. Proc Natl Acad Sci U S A. 2020. PMID: 32601215 Free PMC article.
-
Disulfidptosis: A Novel Prognostic Criterion and Potential Treatment Strategy for Diffuse Large B-Cell Lymphoma (DLBCL).Int J Mol Sci. 2024 Jun 28;25(13):7156. doi: 10.3390/ijms25137156. Int J Mol Sci. 2024. PMID: 39000261 Free PMC article.
-
Distinct or Overlapping Areas of Mitochondrial Thioredoxin 2 May Be Used for Its Covalent and Strong Non-Covalent Interactions with Protein Ligands.Antioxidants (Basel). 2023 Dec 20;13(1):15. doi: 10.3390/antiox13010015. Antioxidants (Basel). 2023. PMID: 38275635 Free PMC article.
-
Per Os Infectivity Factor 5 Identified as a Substrate of P33 in the Baculoviral Disulfide Bond Formation Pathway.J Virol. 2020 Jul 16;94(15):e00615-20. doi: 10.1128/JVI.00615-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32434885 Free PMC article.
References
-
- Braakman I, Bulleid NJ (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 80:71–99. - PubMed
-
- Landeta C, Boyd D, Beckwith J (2018) Disulfide bond formation in prokaryotes. Nat Microbiol 3:270–280. - PubMed
-
- Okumura M, Kadokura H, Inaba K (2015) Structures and functions of protein disulfide isomerase family members involved in proteostasis in the endoplasmic reticulum. Free Radic Biol Med 83:314–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

