Heme oxygenase-1 induction attenuates senescence in chronic obstructive pulmonary disease lung fibroblasts by protecting against mitochondria dysfunction
- PMID: 30341816
- PMCID: PMC6260925
- DOI: 10.1111/acel.12837
Heme oxygenase-1 induction attenuates senescence in chronic obstructive pulmonary disease lung fibroblasts by protecting against mitochondria dysfunction
Abstract
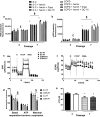
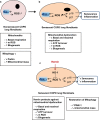
Chronic obstructive pulmonary disease (COPD) is associated with lung fibroblast senescence, a process characterized by an irreversible proliferation arrest associated with secretion of inflammatory mediators. ROS production, known to induce senescence, is increased in COPD fibroblasts and mitochondria dysfunction participates in this process. Among the battery of cellular responses against oxidative stress damage, heme oxygenase (HO)-1 plays a critical role in defending the lung against oxidative stress and inflammation. Therefore, we investigated whether pharmacological induction of HO-1 by chronic hemin treatment attenuates senescence and improves dysfunctional mitochondria in COPD fibroblasts. Fibroblasts from smoker controls (S-C) and COPD patients were isolated from lung biopsies. Fibroblasts were long-term cultured in the presence or absence of hemin, and/or ZnPP or QC-15 (HO-1 inhibitors). Lung fibroblasts from smokers and COPD patients displayed in long-term culture a senescent phenotype, characterized by a reduced replicative capacity, an increased senescence and inflammatory profile. These parameters were significantly higher in senescent COPD fibroblasts which also exhibited decreased mitochondrial activity (respiration, glycolysis, and ATP levels) which led to an increased production of ROS, and mitochondria biogenesis and impaired mitophagy process. Exposure to hemin increased the gene and protein expression level of HO-1 in fibroblasts and diminished ROS levels, senescence, the inflammatory profile and simultaneously rescued mitochondria dysfunction by restoring mitophagy in COPD cells. The effects of hemin were abolished by a cotreatment with ZnPP or QC-15. We conclude that HO-1 attenuates senescence in COPD fibroblasts by protecting, at least in part, against mitochondria dysfunction and restoring mitophagy.
Keywords: chronic obstructive pulmonary disease; fibroblasts; heme oxygenase-1; mitochondria; mitophagy; senescence.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




References
-
- Ahmad, T. , Sundar, I. K. , Lerner, C. A. , Gerloff, J. , Tormos, A. M. , Yao, H. , & Rahman, I. (2015). Impaired mitophagy leads to cigarette smoke stress‐induced cellular senescence: Implications for chronic obstructive pulmonary disease. FASEB Journal, 29, 2912–2929. 10.1096/fj.14-268276 - DOI - PMC - PubMed
-
- Amsellem, V. , Gary‐Bobo, G. , Marcos, E. , Maitre, B. , Chaar, V. , Validire, P. , … Adnot, S. (2011). Telomere dysfunction causes sustained inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 184, 1358–1366. 10.1164/rccm.201105-0802OC - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

