Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges
- PMID: 30342035
- PMCID: PMC6446912
- DOI: 10.1053/j.gastro.2018.10.024
Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges
Abstract
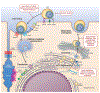
Chronic infection with hepatitis C virus is a major cause of liver disease and hepatocellular carcinoma worldwide. After the discovery of hepatitis C virus 3 decades ago, the identification of the structure of the viral proteins, combined with high-throughput replicon models, enabled the discovery and development of direct-acting antivirals. These agents have revolutionized patient care, with cure rates of more than 90%. We review the status of direct-acting antiviral therapies for hepatitis C virus infection and discuss remaining challenges. We highlight licensed compounds, discuss the potential to shorten therapy even further, and review different options for treatment failure and resistance. We also provide an overview of clinical experience with generic agents and evidence for their efficacy. Finally, we discuss the need for new drugs and outline promising targets for future therapies.
Keywords: Direct-Acting Antivirals; Hepatitis C; Resistance; Treatment Failure.
Copyright © 2019 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- AASLD/IDSA HCV Guidance Panel. The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Present HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Last Updated: May 24, 2018; www.hcvguidelines.org. Hepatology.
-
- Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879–1888. - PubMed
-
- Curry MP, Tapper EB, Bacon B, et al. Effectiveness of 8- or 12-weeks of ledipasvir and sofosbuvir in real-world treatment-naïve, genotype 1 hepatitis C infected patients. Aliment Pharmacol Ther 2017;46:540–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

