Exome sequencing identifies gene variants and networks associated with extreme respiratory outcomes following preterm birth
- PMID: 30342483
- PMCID: PMC6195962
- DOI: 10.1186/s12863-018-0679-7
Exome sequencing identifies gene variants and networks associated with extreme respiratory outcomes following preterm birth
Abstract
Background: Previous studies have identified genetic variants associated with bronchopulmonary dysplasia (BPD) in extremely preterm infants. However, findings with genome-wide significance have been rare, and not replicated. We hypothesized that whole exome sequencing (WES) of premature subjects with extremely divergent phenotypic outcomes could facilitate the identification of genetic variants or gene networks contributing disease risk.
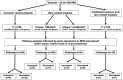
Results: The Prematurity and Respiratory Outcomes Program (PROP) recruited a cohort of > 765 extremely preterm infants for the identification of markers of respiratory morbidity. We completed WES on 146 PROP subjects (85 affected, 61 unaffected) representing extreme phenotypes of early respiratory morbidity. We tested for association between disease status and individual common variants, screened for rare variants exclusive to either affected or unaffected subjects, and tested the combined association of variants across gene loci. Pathway analysis was performed and disease-related expression patterns were assessed. Marginal association with BPD was observed for numerous common and rare variants. We identified 345 genes with variants unique to BPD-affected preterm subjects, and 292 genes with variants unique to our unaffected preterm subjects. Of these unique variants, 28 (19 in the affected cohort and 9 in unaffected cohort) replicate a prior WES study of BPD-associated variants. Pathway analysis of sets of variants, informed by disease-related gene expression, implicated protein kinase A, MAPK and Neuregulin/epidermal growth factor receptor signaling.
Conclusions: We identified novel genes and associated pathways that may play an important role in susceptibility/resilience for the development of lung disease in preterm infants.
Keywords: Bronchopulmonary dysplasia (BPD); Prematurity and respiratory outcomes program (PROP).
Conflict of interest statement
Authors’ information
PROP Investigators:
Barbara Alexander, RN, Claire Chougnet, PhD, Tari Gratton, PA, James M. Greenberg, MD, Cathy Grisby, BSN, CCRC, William Hardie, MD, Alan H. Jobe MD, PhD, Beth Koch, BHS, RRT, RPFT, Karen McDowell, MD, Kelly Thornton BS.
Washington University:
Pamela Bates, CRT, RPFT, RPSGT, Claudia Cleveland, RRT, Thomas Ferkol, MD, Aaron Hamvas, MD, Julie Hoffmann, RN, Mark R. Holland, PhD, James Kemp, MD, Philip T. Levy, MD, Laura Linneman, RN, Jayne Sicard-Su, RN, Gina Simpson, RRT, CPFT, Gautam K. Singh, MD, Barbara Warner, MD.
University of California at San Francisco:
Investigators
Philip L. Ballard, MD, PhD1, Roberta A. Ballard, MD1, David J. Durand, MD2, Eric C. Eichenwald, MD4, Roberta L. Keller, MD1, Amir M. Khan, MD4, Leslie Lusk, MD1, Jeffrey D. Merrill, MD3, Dennis W. Nielson, MD, PhD1, Elizabeth E. Rogers, MD1.
Research Staff
Jeanette M. Asselin, MS, RRT-NPS2, Samantha Balan1, Katrina Burson, RN, BSN4, Cheryl Chapin1, Erna Josiah-Davis, RN, NP3, Carmen Garcia, RN, CCRP4, Hart Horneman1, Rick Hinojosa, BSRT, RRT, CPFT-NPS4, Christopher Johnson, MBA, RRT4, Susan Kelley, RRT1, Karin L. Knowles1, M. Layne Lillie, RN, BSN4, Karen Martin, RN4, Sarah Martin, RN, BSN1, Julie Arldt-McAlister, RN, BSN4, Georgia E. McDavid, RN4, Lori Pacello, RCP2, Shawna Rodgers, RN, BSN4, Daniel K. Sperry, RN4,1
1Department of Pediatrics, University of California San Francisco, San Francisco, CA; 2Children’s Hospital and Research Center Oakland, Oakland, CA.
3Alta Bates Summit Medical Center, Berkeley, CA.
4University of Texas Health Science Center- Houston, Houston, TX.
Vanderbilt University:
Judy Aschner, MD, Amy B Beller BSN, Candice Fike, MD, Scott Guthrie, MD, Tina Hartert, MD, Nathalie Maitre, MD, Paul Moore, MD, Mark O′ Hunt, Theresa J. Rogers, RN, Odessa L. Settles, RN, MSN, CM, Steven Steele, RN, Marshall Summar, MD, Sharon Wadley, BSN, RN, CLS.
University of Rochester-University at Buffalo:
Investigators
Carl D’Angio, MD, Vasanth Kumar, MD, Tom Mariani, PhD, Gloria Pryhuber, MD, Clement Ren, MD, Anne Marie Reynolds, MD, MPH, Rita M. Ryan, MD, Kristin Scheible, MD, Timothy Stevens, MD, MPH.
Technical Staff
Heidie Huyck, BS, Valerie Lunger, MS
Study Staff
Shannon Castiglione, RN, Aimee Horan, LPN, Deanna Maffet, RN, Jane O’Donnell, PNP, Michael Sacilowski, MAT, Tanya Scalise, RN, BSN, Elizabeth Werner, MPH, Jason Zayac, BS Respiratory Therapists and Nurses:
Kim Bordeaux, RRT, Pam Brown, RRT, Julia Epping, AAS, RT, Lisa Flattery-Walsh, RRT, Donna Germuga, RRT, CPFT, Nancy Jenks, RN, Mary Platt, RN, Eileen Popplewell, RRT, Sandra Prentice, CRT.
Duke University:
Kim Ciccio, RN, C. Michael Cotten, M.D., Kim Fisher, Ph.D., Jack Sharp, M.D., Judith A. Voynow, M.D.*.
*Present address, Virginia Commonwealth University.
Indiana University:
Charles Clem, RRT, Stephanie Davis, M.D., Susan Gunn, NNP, CCRC, Lauren Jewett, RN, CCRC Brenda Poindexter, M.D., M.S.#.
#Present address, University of Cincinnati.
Steering Committee Chair:
Lynn M. Taussig, MD, University of Denver.
NHLBI Program Officer:
Carol J. Blaisdell, MD.
University of Pennsylvania Data Coordinating Center:
Scarlett Bellamy, ScD, Maria Blanco, BS, Denise Cifelli, MS, Sara DeMauro, MD, Jonas Ellenberg, PhD, Rui Feng, PhD, Melissa Fernando, MPH, Howard Panitch, MD, Barbara Schmidt, MD, MSc, Pamela Shaw, PhD, Ann Tierney, BA, MS
Ethics approval and consent to participate
Studies at each of the participating PROP sites were IRB-approved and all parents consented to collection and sequencing of infant DNA and collection of clinical data.
Consent for publication
All parents consented to publication of data without identifiers.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, Reynolds AM, Shaw PA, Jobe AH, Prematurity and Respiratory Outcomes Program et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2015;12(12):1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

