Genetic diversity between mouse strains allows identification of the CC027/GeniUnc strain as an orally reactive model of peanut allergy
- PMID: 30342892
- PMCID: PMC7252586
- DOI: 10.1016/j.jaci.2018.10.009
Genetic diversity between mouse strains allows identification of the CC027/GeniUnc strain as an orally reactive model of peanut allergy
Erratum in
-
Corrigenda.J Allergy Clin Immunol. 2021 Jun;147(6):2394. doi: 10.1016/j.jaci.2021.03.007. J Allergy Clin Immunol. 2021. PMID: 34092355 No abstract available.
Abstract
Background: Improved animal models are needed to understand the genetic and environmental factors that contribute to food allergy.
Objective: We sought to assess food allergy phenotypes in a genetically diverse collection of mice.
Methods: We selected 16 Collaborative Cross (CC) mouse strains, as well as the classic inbred C57BL/6J, C3H/HeJ, and BALB/cJ strains, for screening. Female mice were sensitized to peanut intragastrically with or without cholera toxin and then challenged with peanut by means of oral gavage or intraperitoneal injection and assessed for anaphylaxis. Peanut-specific immunoglobulins, T-cell cytokines, regulatory T cells, mast cells, and basophils were quantified.
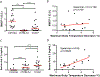
Results: Eleven of the 16 CC strains had allergic reactions to intraperitoneal peanut challenge, whereas only CC027/GeniUnc mice reproducibly experienced severe symptoms after oral food challenge (OFC). CC027/GeniUnc, C3H/HeJ, and C57BL/6J mice all mounted a TH2 response against peanut, leading to production of IL-4 and IgE, but only the CC027/GeniUnc mice reacted to OFC. Orally induced anaphylaxis in CC027/GeniUnc mice was correlated with serum levels of Ara h 2 in circulation but not with allergen-specific IgE or mucosal mast cell protease 1 levels, indicating systemic allergen absorption is important for anaphylaxis through the gastrointestinal tract. Furthermore, CC027/GeniUnc, but not C3H/HeJ or BALB/cJ, mice can be sensitized in the absence of cholera toxin and react on OFC to peanut.
Conclusions: We have identified and characterized CC027/GeniUnc mice as a strain that is genetically susceptible to peanut allergy and prone to severe reactions after OFC. More broadly, these findings demonstrate the untapped potential of the CC population in developing novel models for allergy research.
Keywords: Ara h 2; Collaborative Cross; anaphylaxis; food allergy; mouse model; peanut allergy.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

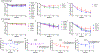

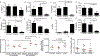

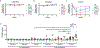
References
-
- Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. The Journal of allergy and clinical immunology. 2010;125(6):1322–6. - PubMed
-
- Avery NJ, King RM, Knight S, Hourihane JO. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol. 2003;14(5):378–82. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291–307; quiz 8. - PubMed
-
- Iweala OI, Burks AW. Food Allergy: Our Evolving Understanding of Its Pathogenesis, Prevention, and Treatment. Current allergy and asthma reports. 2016;16(5):37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

