Appraisal of systemic inflammation and diagnostic markers in a porcine model of VAP: secondary analysis from a study on novel preventive strategies
- PMID: 30343359
- PMCID: PMC6195872
- DOI: 10.1186/s40635-018-0206-1
Appraisal of systemic inflammation and diagnostic markers in a porcine model of VAP: secondary analysis from a study on novel preventive strategies
Abstract
Background: We previously evaluated the efficacy of a ventilatory strategy to achieve expiratory flow bias and positive end-expiratory pressure (EFB + PEEP) or the Trendelenburg position (TP) for the prevention of ventilator-associated pneumonia (VAP). These preventive measures were aimed at improving mucus clearance and reducing pulmonary aspiration of bacteria-laden oropharyngeal secretions. This secondary analysis is aimed at evaluating the effects of aforementioned interventions on systemic inflammation and to substantiate the value of clinical parameters and cytokines in the diagnosis of VAP.
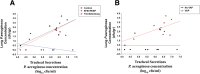
Methods: Twenty female pigs were randomized to be positioned in the semirecumbent/prone position, and ventilated with duty cycle 0.33 and without PEEP (control); positioned as in the control group, PEEP 5 cmH2O, and duty cycle to achieve expiratory flow bias (EFB+PEEP); ventilated as in the control group, but in the Trendelenburg position (Trendelenburg). Following randomization, P. aeruginosa was instilled into the oropharynx. Systemic cytokines and tracheal secretions P. aeruginosa concentration were quantified every 24h. Lung biopsies were collected for microbiological confirmation of VAP.
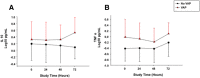
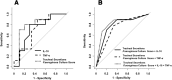
Results: In the control, EFB + PEEP, and Trendelenburg groups, lung tissue Pseudomonas aeruginosa concentration was 2.4 ± 1.5, 1.9 ± 2.1, and 0.3 ± 0.6 log cfu/mL, respectively (p = 0.020). Whereas, it was 2.4 ± 1.9 and 0.6 ± 0.9 log cfu/mL in animals with or without VAP (p < 0.001). Lower levels of interleukin (IL)-1β (p = 0.021), IL-1RA (p < 0.001), IL-4 (p = 0.005), IL-8 (p = 0.008), and IL-18 (p = 0.050) were found in Trendelenburg animals. VAP increased IL-10 (p = 0.035), tumor necrosis factor-α (p = 0.041), and endotracheal aspirate (ETA) P. aeruginosa concentration (p = 0.024). A model comprising ETA bacterial burden, IL-10, and TNF-α yielded moderate discrimination for the diagnosis of VAP (area of the receiver operating curve 0.82, 95% CI 0.61-1.00).
Conclusions: Our findings demonstrate anti-inflammatory effects associated with the Trendelenburg position. In this reliable model of VAP, ETA culture showed good diagnostic accuracy, whereas systemic IL-10 and TNF-α marginally improved accuracy. Further clinical studies will be necessary to confirm clinical value of the Trendelenburg position as a measure to hinder inflammation during mechanical ventilation and significance of systemic IL-10 and TNF-α in the diagnosis of VAP.
Keywords: Inflammation; Interleukin; Mechanical ventilation; Semirecumbent; Trendelenburg; Ventilator-associated pneumonia.
Conflict of interest statement
Ethics approval and consent to participate
The Institutional Ethics Committee evaluated and approved our study protocol: Dr. Jordi Alberch Vie; Álvaro Gimeno Sandig; Raquel Corral Vistué; Dr. Garikoitz Azkona Mendoza; Dr. Victor Fernández Dueñas; Dr. Jordi Guinea Mejías; Dr. Francesc López Soriano; Dr. Carmen Navarro Aragay; Dr. Francisco José Pérez Can; Dr. Montserrat Rigol Muixart; and Dr. Teresa Rodrigo Calduch.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. - DOI - PMC - PubMed
-
- Torres Antoni, Niederman Michael S., Chastre Jean, Ewig Santiago, Fernandez-Vandellos Patricia, Hanberger Hakan, Kollef Marin, Li Bassi Gianluigi, Luna Carlos M., Martin-Loeches Ignacio, Paiva J. Artur, Read Robert C., Rigau David, Timsit Jean François, Welte Tobias, Wunderink Richard. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. European Respiratory Journal. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

