A pooled analysis of individual patient data from National Clinical Trials Network clinical trials of concurrent chemoradiotherapy for limited-stage small cell lung cancer in elderly patients versus younger patients
- PMID: 30343497
- PMCID: PMC6340765
- DOI: 10.1002/cncr.31813
A pooled analysis of individual patient data from National Clinical Trials Network clinical trials of concurrent chemoradiotherapy for limited-stage small cell lung cancer in elderly patients versus younger patients
Abstract
Background: Platinum and etoposide with thoracic radiation followed by prophylactic cranial irradiation constitute the standard treatment for limited-stage small cell lung cancer (LS-SCLC). Many patients with LS-SCLC are elderly with comorbidities.
Methods: Individual patient data were collected from 11 phase 2 or 3 trials for LS-SCLC conducted by the National Clinical Trials Network and activated from 1990 to 2010. The primary endpoint was overall survival (OS); the secondary endpoints were progression-free survival (PFS), the rate of severe adverse events, and off-treatment reasons. The outcomes were compared for patients 70 years old or older (elderly patients) and patients younger than 70 years (younger patients).
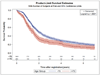
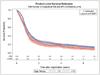
Results: Individual patient data from 1049 younger patients (81%) and 254 elderly patients (19%) were analyzed. In the multivariate model, elderly patients, in comparison with younger patients, had worse OS (hazard ratio [HR], 1.38; 95% confidence interval [CI], 1.18-1.63; median OS for elderly patients, 17.8 months; OS for younger patients, 23.5 months) and worse PFS (HR, 1.19; 95% CI, 1.03-1.39; median PFS for elderly patients, 10.6 months; median PFS for younger patients, 12.3 months). Elderly patients, in comparison with younger patients, experienced more grade 5 adverse events (8% vs 3%; P < .01) and more grade 3 or higher dyspnea (11% vs 7%; P = .03) but less grade 3 or higher esophagitis/dysphagia (14% vs 19%; P = .04) and less grade 3 or higher vomiting (11% vs 17%; P = .01). Elderly patients completed treatment less often, discontinued treatment because of adverse events and patient refusal more frequently, and died during treatment more frequently.
Conclusions: Elderly patients with LS-SCLC have worse PFS and OS and more difficulty in tolerating therapy. Future trials should incorporate assessments of elderly patients, novel monitoring of adverse events, and more tolerable radiation and systemic therapies.
Keywords: adverse events related to age; chemotherapy; clinical trial; small cell lung cancer; thoracic radiation therapy.
© 2018 American Cancer Society.
Conflict of interest statement
Conflict of interest:
The following authors do not report any conflicts of interest: Stinchcombe, Cohen, Pang, Le, Fan, Bogart, Vokes, Horn, Edelman, Komaki, Schild, Bogart, Thomas, and Wang
Dr. Ganti reports grants and personal fees from Pfizer, grants from New Link Genetics, personal fees from Ariad Pharmaceuticals, grants from Amgen, grants from AstraZeneca, grants from Merck, grants from Janssen, grants from Bristol-Myers Squibb, personal fees from Biodesix, outside the submitted work.
Figures
Similar articles
-
Association of Chemoradiotherapy With Outcomes Among Patients With Stage I to II vs Stage III Small Cell Lung Cancer: Secondary Analysis of a Randomized Clinical Trial.JAMA Oncol. 2019 Mar 1;5(3):e185335. doi: 10.1001/jamaoncol.2018.5335. Epub 2019 Mar 14. JAMA Oncol. 2019. PMID: 30520977 Free PMC article. Clinical Trial.
-
Six versus four or five cycles of first-line etoposide and platinum-based chemotherapy combined with thoracic radiotherapy in patients with limited-stage small-cell lung cancer: A propensity score-matched analysis of a prospective randomized trial.Cancer Med. 2024 Apr;13(8):e7215. doi: 10.1002/cam4.7215. Cancer Med. 2024. PMID: 38659392 Free PMC article. Clinical Trial.
-
A pooled analysis of limited-stage small-cell lung cancer patients treated with induction chemotherapy followed by concurrent platinum-based chemotherapy and 70 Gy daily radiotherapy: CALGB 30904.J Thorac Oncol. 2013 Aug;8(8):1043-9. doi: 10.1097/JTO.0b013e318293d8a4. J Thorac Oncol. 2013. PMID: 23715301 Free PMC article. Clinical Trial.
-
A retrospective evaluation of therapeutic efficacy and safety of chemoradiotherapy in older patients (aged ≥ 75 years) with limited-disease small cell lung cancer: insights from two institutions and review of the literature.Radiol Oncol. 2024 Sep 15;58(3):432-443. doi: 10.2478/raon-2024-0054. eCollection 2024 Sep 1. Radiol Oncol. 2024. PMID: 39287161 Free PMC article. Review.
-
Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer.J Clin Oncol. 2022 Feb 20;40(6):661-670. doi: 10.1200/JCO.21.01639. Epub 2022 Jan 5. J Clin Oncol. 2022. PMID: 34985935 Free PMC article. Review.
Cited by
-
A prognostic nomogram to predict survival in elderly patients with small-cell lung cancer: a large population-based cohort study and external validation.BMC Cancer. 2022 Dec 6;22(1):1271. doi: 10.1186/s12885-022-10333-9. BMC Cancer. 2022. PMID: 36474197 Free PMC article. Clinical Trial.
-
Establishment of a prognostic nomogram for elderly patients with limited-stage small cell lung cancer receiving radiotherapy.Sci Rep. 2024 May 25;14(1):11990. doi: 10.1038/s41598-024-62533-x. Sci Rep. 2024. PMID: 38796503 Free PMC article.
-
Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients.Thorac Cancer. 2021 Jul;12(13):1943-1951. doi: 10.1111/1759-7714.13977. Epub 2021 May 9. Thorac Cancer. 2021. PMID: 33969619 Free PMC article.
-
Characteristics and clinical outcomes of patients with nonsmoking small cell lung cancer in Korea.BMC Pulm Med. 2022 May 18;22(1):200. doi: 10.1186/s12890-022-01989-x. BMC Pulm Med. 2022. PMID: 35585538 Free PMC article.
-
A retrospective study on the impact of radiotherapy on the survival outcomes of small cell lung cancer patients based on the SEER database.Sci Rep. 2024 Jul 5;14(1):15552. doi: 10.1038/s41598-024-65314-8. Sci Rep. 2024. PMID: 38969694 Free PMC article.
References
-
- National Cancer Institute: SEER stat fact sheets: Lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html-accesse 3/19/2018.
-
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24: 4539–4544. - PubMed
-
- Fruh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6: vi99–105. - PubMed
-
- Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer (correction). Annals of Oncology. 2014;25: 1672. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical