Leukotriene receptor antagonists for eczema
- PMID: 30343498
- PMCID: PMC6517006
- DOI: 10.1002/14651858.CD011224.pub2
Leukotriene receptor antagonists for eczema
Abstract
Background: Eczema is a common, chronic, inflammatory skin condition that is frequently associated with atopic conditions, including asthma. Leukotriene receptor antagonists (LTRAs) have a corticosteroid-sparing role in asthma, but their role in eczema remains controversial. Currently available topical therapies for eczema are often poorly tolerated, and use of systemic agents is restricted by their adverse effect profile. A review of alternative treatments was therefore warranted.
Objectives: To assess the possible benefits and harms of leukotriene receptor antagonists for eczema.
Search methods: We searched the following databases to September 2017: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and the GREAT database. We also searched five trial registries, and handsearched the bibliographies of all extracted studies for further relevant trials.
Selection criteria: Randomised controlled trials of LTRAs alone or in combination with other (topical or systemic) treatments compared with other treatments alone such as topical corticosteroids or placebo for eczema in the acute or chronic (maintenance) phase of eczema in adults and children.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane. The primary outcome measures were change in disease severity, long-term symptom control, and adverse effects of treatment. Secondary outcomes were change in corticosteroid requirement, reduction of pruritis, quality of life, and emollient requirement. We used GRADE to assess the quality of the evidence for each outcome.
Main results: Only five studies (including a total of 202 participants) met the inclusion criteria, all of which assessed oral montelukast; hence, we found no studies assessing other LTRAs. Treatment ranged from four to eight weeks, and outcomes were assessed at the end of treatment; therefore, we could only report short-term measurements (defined as less than three months follow-up from baseline). Montelukast dosing was 10 mg for adults (age 14 years and above) and 5 mg for children (age 6 years to 14 years). One study included children (aged 6 years and above) among their participants, while the remaining studies only included adults (participant age ranged from 16 to 70 years). The participants were diagnosed with moderate-to-severe eczema in four studies and moderate eczema in one study. The study setting was unclear in two studies, multicentre in two studies, and single centre in one study; the studies were conducted in Europe and Bangladesh. Two studies were industry funded. The comparator was placebo in three studies and conventional treatment in two studies. The conventional treatment comparator was a combination of antihistamines and topical corticosteroids (plus oral antibiotics in one study).Four of the studies did not adequately describe their randomisation or allocation concealment method and were considered as at unclear risk of selection bias. Only one study was at low risk of performance and detection bias. However, we judged all studies to be at low risk of attrition and reporting bias.We found no evidence of a difference in disease severity of moderate-to-severe eczema after short-term use of montelukast (10 mg) when compared with placebo. The outcome was assessed using the modified EASI (Eczema Area and Severity Index) score and SASSAD (Six Area, Six Sign Atopic Dermatitis) severity score (standardised mean difference 0.29, with a positive score showing montelukast is favoured, 95% confidence interval (CI) -0.23 to 0.81; 3 studies; n = 131; low-quality evidence).When short-term montelukast (10 mg) treatment was compared with conventional treatment in one study, the mean improvement in severity of moderate-to-severe eczema was greater in the intervention group (measured using SCORAD (SCORing of Atopic Dermatitis) severity index) (mean difference 10.57, 95% CI 4.58 to 16.56; n = 31); however, another study of 32 participants found no significant difference between groups using the same measure (mean improvement was 25.2 points with montelukast versus 23.9 points with conventional treatment; no further numerical data provided). We judged the quality of the evidence as very low for this outcome, meaning the results are uncertain.All studies reported their adverse event rate during treatment. Four studies (136 participants) reported no adverse events. In one study of 58 participants with moderate eczema who received montelukast 10 mg (compared with placebo), there was one case of septicaemia and one case of dizziness reported in the intervention group, both resulting in study withdrawal, although whether these effects were related to the medication is unclear. Mild side effects (e.g. headache and mild gastrointestinal disturbances) were also noted, but these were fairly evenly distributed between the montelukast and placebo groups. The quality of evidence for this outcome was low.No studies specifically evaluated emollient requirement or quality of life. One study that administered treatment for eight weeks specifically evaluated pruritus improvement at the end treatment and topical corticosteroid use during treatment. We found no evidence of a difference between montelukast (10 mg) and placebo for both outcomes (low-quality evidence, n = 58). No other study assessed these outcomes.
Authors' conclusions: The findings of this review are limited to montelukast. There was a lack of evidence addressing the review question, and the quality of the available evidence for most of the measured outcomes was low. Some primary and secondary outcomes were not addressed at all, including long-term control.We found no evidence of a difference between montelukast (10 mg) and placebo on disease severity, pruritus improvement, and topical corticosteroid use. Very low-quality evidence means we are uncertain of the effect of montelukast (10 mg) compared with conventional treatment on disease severity. Participants in only one study reported adverse events, which were mainly mild (low-quality evidence).There is no evidence that LTRA is an effective treatment for eczema. Serious limitations were that all studies focused on montelukast and only included people with moderate-to-severe eczema, who were mainly adults; and that each outcome was evaluated with a small sample size, if at all.Further large randomised controlled trials, with a longer treatment duration, of adults and children who have eczema of all severities may help to evaluate the effect of all types of LTRA, especially on eczema maintenance.
Conflict of interest statement
Masaki Futamura: "I have received lecture honoraria for speaking about atopic eczema from Maruho Co., Ltd. and TAIHO Pharmaceutical Co., Ltd, and for speaking about treatment of asthma from MSD KK and KYORIN Pharmaceutical Co., Ltd. MSD K.K. is a subsidiary of Merck & Co., Inc. and MSD K.K. and KYORIN Pharmaceutical Co., Ltd. sell a leukotriene receptor antagonist, Singlair or Kipres." Leila Ferguson: Nothing to declare. Efstratios Vakirlis: "I have received lecture honoraria from Meda Pharmaceuticals, LEO Pharma, Pierre Fabre, and La Roche Posay as an invited speaker for atopic dermatitis. I have received lecture honoraria from Novartis, Janssen, and Genesis as an invited speaker for psoriasis." Reiji Kojima: Nothing to declare. Hatoko Sasaki: Nothing to declare. Amanda Roberts: Nothing to declare. Rintaro Mori: Nothing to declare. Antonia Lloyd‐Lavery (clinical referee): "I have acted as co‐investigator for three phase 3 clinical trials investigating different treatments for moderate‐severe atopic eczema in adults (Atopix – OC459; Treble – Lebrikizumab; Café – Dupilumab)."
Masaki Futamura, lead author on the protocol of this Cochrane Review, became conflicted in terms of Cochrane’s commercial sponsorship policy and has been replaced by Leila Ferguson, as lead author. Leila Ferguson has no relevant financial conflict of interest, and in the judgement of Cochrane Skin’s editorial team, she has made a contribution equal to Masaki Futamura, which justifies her position as first author. This change has been discussed with and approved by the Funding Arbiters.
Figures


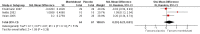
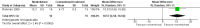
Update of
References
References to studies included in this review
Capella 2001 {published data only}
-
- Capella GL, Frigerio E, Altomare G. A randomized trial of leukotriene receptor antagonist montelukast in moderate‐to‐severe atopic dermatitis of adults. European Journal of Dermatology 2001;11(3):209‐13. [CENTRAL: CN‐00347986; PUBMED: 11358726] - PubMed
Friedmann 2007 {published data only}
-
- Friedmann PS, Palmer R, Tan E, Ogboli M, Barclay G, Hotchkiss K, et al. A double‐blind, placebo‐controlled trial of montelukast in adult atopic eczema. Clinical and Experimental Allergy 2007;37(10):1536‐40. [CENTRAL: CN‐00612029; PUBMED: 17850382] - PubMed
Nettis 2002 {published data only}
-
- Nettis E, Pannofino A, Fanelli M, Ferrannini A, Tursi A. Efficacy and tolerability of montelukast as a therapeutic agent for severe atopic dermatitis in adults. Acta dermato‐venereologica 2002;82(4):297‐8. [CENTRAL: CN‐00422152; EMBASE: 35015053] - PubMed
Rahman 2006 {published data only}
-
- Rahman ML, Choudhury AM, Islam MM. Effectiveness of montelukast in the treatment of atopic dermatitis. Mymensingh Medical Journal 2006;15(1):85‐8. [CENTRAL: CN‐00562538; PUBMED: 16467770] - PubMed
Veien 2005 {published data only}
-
- Veien NK, Busch‐Sorensen M, Stausbol‐Gron B. Montelukast treatment of moderate to severe atopic dermatitis in adults: a randomized, double‐blind, placebo‐controlled trial. Journal of the American Academy of Dermatology 2005;53(1):147‐9. [CENTRAL: CN‐00551665; PUBMED: 15965438] - PubMed
References to studies excluded from this review
Boguniewicz 2003 {published data only}
-
- Boguniewicz M, Eichenfield LF, Hultsch T. Current management of atopic dermatitis and interruption of the atopic march. Journal of Allergy and Clinical Immunology 2003;112(6 Suppl):S140‐50. [PUBMED: 14657844] - PubMed
Concha 2003 {published data only}
-
- Concha del Rio LE, Arroyave CM. A leukotriene antagonist (montelukast) in the treatment of atopic dermatitis in pediatric patients [Antagonista de un leucotrienno (montelukast) en el tratamiento de dermatitis atopica en pacientes pediatricos]. Revista Alergia Mexico 2003;50(5):187‐91. [PUBMED: 14631590] - PubMed
Ehlayel 2007 {published data only}
-
- Ehlayel MS, Bener A, Sabbah A. Montelukast treatment in children with moderately severe atopic dermatitis. European Annals of Allergy & Clinical Immunology 2007;39(7):232‐6. [PUBMED: 18236999] - PubMed
Ehlayel 2008 {published data only}
-
- Ehlayel MS, Bener A. Does montelukast reduce the treatment cost in children with moderately severe atopic dermatitis. Current Pediatric Research 2008;12(1‐2):1‐4. [EMBASE: 352277706]
Goh 2016 {published data only}
-
- Goh A, Lowe A, Zallmann, Su J. The effectiveness of montelukast as adjunct treatment among children with atopic dermatitis: An open‐label, randomized controlled trial. Australasian Journal of Dermatology 2016;57:52. [CENTRAL: CN‐01160198]
Holme 2013 {published data only}
-
- Holme H, Winckworth LC. Montelukast can reduce the severity and extent of atopic dermatitis. Journal of Paediatrics and Child Health 2013;49(5):412‐5. [PUBMED: 23647765] - PubMed
Jackson 2004 {published data only}
-
- Jackson CM, Lee DK, Lipworth BJ. The effects of butterbur on the histamine and allergen cutaneous response. Annals of Allergy, Asthma, & Immunology 2004;92(2):250‐4. [PUBMED: 14989395] - PubMed
Jeon 2016 {published data only}
Lehtimaki 2009 {published data only}
-
- Lehtimaki L, Petays T, Haahtela T. Montelukast is not effective in controlling allergic symptoms outside the airways: a randomised double‐blind placebo‐controlled crossover study. International Archives of Allergy & Immunology 2009;149(2):150‐3. [PUBMED: 19127072] - PubMed
NCT00903357 {unpublished data only}
-
- NCT00903357. The effectiveness of montelukast on atopic dermatitis in Koreans [A double blind, randomized, crossover study to compare the effectiveness of montelukast on atopic dermatitis in Koreans]. clinicaltrials.gov/ct2/show/NCT00903357 Date first received: 14 May 2009.
Pei 2001 {published data only}
-
- Pei AY, Chan HH, Leung TF. Montelukast in the treatment of children with moderate‐to‐severe atopic dermatitis: a pilot study. Pediatric Allergy and Immunology 2001;12(3):154‐8. [PUBMED: 11473680] - PubMed
Woodmansee 1999 {published data only}
-
- Woodmansee DP, Simon RA. A pilot study examining the role of zileuton in atopic dermatitis. Annals of Allergy, Asthma, & Immunology 1999;83(6 Pt 1):548‐52. [PUBMED: 10619348] - PubMed
Yanase 2001 {published data only}
-
- Yanase DJ, David‐Bajar K. The leukotriene antagonist montelukast as a therapeutic agent for atopic dermatitis. Journal of the American Academy of Dermatology 2001;44(1):89‐93. [PUBMED: 11148482] - PubMed
Zouboulis 2003 {published data only}
-
- Zouboulis Ch C. Leukotriene antagonists in atopic diseases and acne [Leukotrienantagonisten bei Atopischen Erkrankungen und Akne]. Aktuelle Dermatologie 2003;29(10):419‐25. [EMBASE: 37376885]
References to studies awaiting assessment
Craig 2002 {published data only}
-
- Craig TJ, Correale C, Chinchilli V, Lehman E, Mende C, Longernecker A, et al. The effects of montelukast on atopic dermatitis (AD): a placebo controlled, double blind study. Journal of Allergy, Asthma and Immunolgy 2002;109(Suppl 1):S160. [CENTRAL: CN‐00483619]
Melamed 2010 {published data only}
-
- Melamed IR, Robinson L, Heffron M. The benefit of montelukast in atopic dermatitis induced by food allergies. Journal of Allergy and Clinical Immunology 2010;125(2 Suppl 1):AB93. [EMBASE: 70155473]
Additional references
Adamek‐Guzik 2002
-
- Adamek‐Guzik T, Guzik TJ, Czerniawska‐Mysik G, Korpanty G, Mastalerz L, Radwan J, et al. Urinary leukotriene levels are increased during exacerbation of atopic eczema/dermatitis syndrome. Relation to clinical status. Allergy 2002;57(8):732‐6. [PUBMED: 12121194] - PubMed
Akdis 2006
-
- Akdis CA, Akdis M, Bieber T, Bindslev‐Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. Journal of Allergy and Clinical Immunology 2006;118(1):152‐69. [PUBMED: 16815151] - PubMed
Arkwright 2013
-
- Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult‐to‐treat atopic dermatitis. Journal of Allergy and Clinical Immunology. In practice 2013;1(2):142‐51. [PUBMED: 24565453] - PubMed
Artik 2003
-
- Artik S, Ruzicka T. Complementary therapy for atopic eczema and other allergic skin diseases. Dermatologic Therapy 2003;16(2):150‐63. [PUBMED: 12919117] - PubMed
Ashcroft 2007
Asthma Canada 2018
-
- Asthma Canada. Leukotriene receptor antagonists (LTRAs). www.asthma.ca/get‐help/asthma‐3/treatment/leukotriene/ (accessed 29 August 2018).
Bass 2015
Bisgaard 2005
-
- Bisgaard H, Zielen S, Garcia‐Garcia ML, Johnston SL, Gilles L, Menten J, et al. Montelukast reduces asthma exacerbations in 2‐ to 5‐year‐old children with intermittent asthma. American Journal of Respiratory & Critical Care Medicine 2005;171(4):315‐22. [PUBMED: 15542792] - PubMed
Callen 2007
-
- Callen J, Chamlin S, Eichenfield LF, Ellis C, Girardi M, Goldfarb M, et al. A systematic review of the safety of topical therapies for atopic dermatitis. British Journal of Dermatology 2007;156(2):203‐21. [PUBMED: 17223859] - PubMed
Chauhan 2012
CONSORT 2010
Darsow 2005
-
- Darsow U, Lubbe J, Taieb A, Seidenari S, Wollenberg A, Calza AM, et al. Position paper on diagnosis and treatment of atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 2005;19(3):286‐95. [PUBMED: 15857453] - PubMed
Egger 1997
Eichenfield 2014
-
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology 2014;71(1):116‐32. [PUBMED: 24813302] - PMC - PubMed
Eichenfield 2014a
GINA 2014
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. www.ginasthma.org/local/uploads/files/GINA_Report_2014_Jun11.pdf (accessed 15 July 2014).
Gupta 2004
-
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clinical & Experimental Allergy 2004;34(4):520‐6. [PUBMED: 15080802] - PubMed
Guttman‐Yassky 2013
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holm 2006
-
- Holm EA, Esmann S, Jemec GB. The handicap caused by atopic dermatitis‐‐sick leave and job avoidance. Journal of the European Academy of Dermatology and Venereology 2006;20(3):255‐9. [PUBMED: 16503882] - PubMed
Iversen 1994
-
- Iversen L, Kristensen P, Gron B, Ziboh VA, Kragballe K. Human epidermis transforms exogenous leukotriene A4 into peptide leukotrienes: possible role in transcellular metabolism. Archives of Dermatological Research 1994;286(5):261‐6. [PUBMED: 7914721] - PubMed
Johansson 2004
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy & Clinical Immunology 2004;113(5):832‐6. [PUBMED: 15131563] - PubMed
Joos 2008
-
- Joos S, Miksch A, Szecsenyi J, Wieseler B, Grouven U, Kaiser T, et al. Montelukast as add‐on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: a systematic review. Thorax 2008;63(5):453‐62. [PUBMED: 18443162] - PubMed
Katayama 2014
-
- Katayama I, Kohno Y, Akiyama K, Aihara M, Kondo N, Saeki H, et al. Japanese Guideline for Atopic Dermatitis 2014. Allergology International 2014;63(3):377‐98. [PUBMED: 25178178] - PubMed
Katsarou 2011
-
- Katsarou A, Armenaka M. Atopic dermatitis in older patients: particular points. Journal of the European Academy of Dermatology and Venereology 2011;25(1):12‐8. [PUBMED: 20569298] - PubMed
Luoma 1983
-
- Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years. A prospective study of 543 newborns. Allergy 1983;38(5):339–46. [PUBMED: 6614406] - PubMed
Moore 2006
-
- Moore K, David TJ, Murray CS, Child F, Arkwright PD. Effect of childhood eczema and asthma on parental sleep and well‐being: a prospective comparative study. British Journal of Dermatology 2006;154(3):514‐8. [PUBMED: 16445784] - PubMed
NICE 2007
-
- National Institute for Health and Clinical Excellence. Atopic eczema in children. Management of atopic eczema in children from birth up to the age of 12 years. NICE clinical guideline 57 2007. [www.nice.org.uk/nicemedia/pdf/cg057niceguideline.pdf]
Odhiambo 2009
-
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. Journal of Allergy & Clinical Immunology 2009;124(6):1251‐8. [PUBMED: 20004783] - PubMed
Oymar 2005
-
- Oymar K, Aksnes L. Increased levels of urinary leukotriene E4 in children with severe atopic eczema/dermatitis syndrome. Allergy 2005;60(1):86‐9. [PUBMED: 15575936] - PubMed
Papadopoulos 2012
Reid 1995
-
- Reid P, Lewis‐Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clinical and Experimental Dermatology 1995;20(1):38‐41. [PUBMED: 7671394] - PubMed
Ring 2012
-
- Ring J, Alomar A, Bieber T, Deleuran M, Fink‐Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. Journal of the European Academy of Dermatology and Venereology 2012;26(8):1045‐60. [PUBMED: 22805051] - PubMed
Ring 2012a
-
- Ring J, Alomar A, Bieber T, Deleuran M, Fink‐Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. Journal of the European Academy of Dermatology and Venereology 2012;26(9):1176‐93. [PUBMED: 22813359] - PubMed
Robertson 2007
-
- Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, et al. Short‐course montelukast for intermittent asthma in children: a randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 2007;175(4):323‐9. [PUBMED: 17110643] - PubMed
Roekevish 2014
-
- Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate‐to‐severe atopic dermatitis: a systematic review. Journal of Allergy & Clinical Immunology 2014;133(2):429‐38. [PUBMED: 24269258] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. gdt.gradepro.org/app/handbook/handbook.html (accessed prior to 29 August 2018).
Sher 2012
-
- Sher LG, Chang J, Patel IB, Balkrishnan R, Fleischer AB Jr. Relieving the pruritus of atopic dermatitis: a meta‐analysis. Acta Dermato‐Venereologica 2012;92(5):455‐61. [PUBMED: 22773026] - PubMed
Sidbury 2014
-
- Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. Journal of the American Academy of Dermatology 2014;71(2):327‐49. [PUBMED: 24813298] - PMC - PubMed
Simon 2011
-
- Simon D. Systemic therapy of atopic dermatitis in children and adults. Current Problems in Dermatology 2011;41:156‐64. [MEDLINE: ] - PubMed
Spergel 2010
-
- Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunology & Allergy Clinics of North America 2010;30(3):269‐80. [PUBMED: 20670812] - PubMed
Stone 2002
-
- Stone KD. Atopic diseases of childhood. Current Opinion in Pediatrics 2002;14(5):634‐46. [PUBMED: 12352260] - PubMed
Thomsen 2007
-
- Thomsen SF, Ulrik CS, Kyvik KO, Hjelmborg Jv, Skadhauge LR, Steffensen I, et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy and Asthma Proceedings 2007;28(5):535‐9. [PUBMED: 18034971] - PubMed
Thomsen 2015
van Zuuren 2017
Watts 2012
WebPlotDigitizer [Computer program]
-
- Ankit Rohatgi. WebPlotDigitizer. Version 3.12. Austin, Texas, USA, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

