Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results From a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients With Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors
- PMID: 30343531
- PMCID: PMC6593790
- DOI: 10.1002/art.40753
Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results From a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients With Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors
Abstract
Objective: To investigate the efficacy and safety of ixekizumab in patients with active radiographic axial spondyloarthritis (SpA) and prior inadequate response to or intolerance of 1 or 2 tumor necrosis factor inhibitors (TNFi).
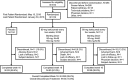
Methods: In this phase III randomized, double-blind, placebo-controlled trial, adult patients with an inadequate response to or intolerance of 1 or 2 TNFi and an established diagnosis of axial SpA (according to the Assessment of SpondyloArthritis international Society [ASAS] criteria for radiographic axial SpA, with radiographic sacroiliitis defined according to the modified New York criteria and ≥1 feature of SpA) were recruited and randomized 1:1:1 to receive placebo or 80-mg subcutaneous ixekizumab every 2 weeks (IXEQ2W) or 4 weeks (IXEQ4W), with an 80-mg or 160-mg starting dose. The primary end point was 40% improvement in disease activity according to the ASAS criteria (ASAS40) at week 16. Secondary outcomes and safety were also assessed.
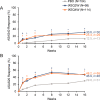
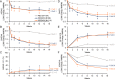
Results: A total of 316 patients were randomized to receive placebo (n = 104), IXEQ2W (n = 98), or IXEQ4W (n = 114). At week 16, significantly higher proportions of IXEQ2W patients (n = 30 [30.6%]; P = 0.003) or IXEQ4W patients (n = 29 [25.4%]; P = 0.017) had achieved an ASAS40 response versus the placebo group (n = 13 [12.5%]), with statistically significant differences reported as early as week 1 with ixekizumab treatment. Statistically significant improvements in disease activity, function, quality of life, and spinal magnetic resonance imaging-evident inflammation were observed after 16 weeks of ixekizumab treatment versus placebo. Treatment-emergent adverse events (AEs) with ixekizumab treatment were more frequent than with placebo. Serious AEs were similar across treatment arms. One death was reported (IXEQ2W group).
Conclusion: Ixekizumab treatment for 16 weeks in patients with active radiographic axial SpA and previous inadequate response to or intolerance of 1 or 2 TNFi yields rapid and significant improvements in the signs and symptoms of radiographic axial SpA versus placebo.
Trial registration: ClinicalTrials.gov NCT02696798.
© 2018 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

References
-
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. - PubMed
-
- Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379–90. - PubMed
-
- Van der Heijde D, Ramiro S, Landewe R, Baraliakos X, van den Bosch F, Sepriano A, et al. 2016 update of the ASAS‐EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. - PubMed
-
- Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

