Tissue-specific pathways and networks underlying sexual dimorphism in non-alcoholic fatty liver disease
- PMID: 30343673
- PMCID: PMC6196429
- DOI: 10.1186/s13293-018-0205-7
Tissue-specific pathways and networks underlying sexual dimorphism in non-alcoholic fatty liver disease
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) encompasses benign steatosis and more severe conditions such as non-alcoholic steatohepatitis (NASH), cirrhosis, and liver cancer. This chronic liver disease has a poorly understood etiology and demonstrates sexual dimorphisms. We aim to examine the molecular mechanisms underlying sexual dimorphisms in NAFLD pathogenesis through a comprehensive multi-omics study. We integrated genomics (DNA variations), transcriptomics of liver and adipose tissue, and phenotypic data of NAFLD derived from female mice of ~ 100 strains included in the hybrid mouse diversity panel (HMDP) and compared the NAFLD molecular pathways and gene networks between sexes.
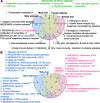
Results: We identified both shared and sex-specific biological processes for NAFLD. Adaptive immunity, branched chain amino acid metabolism, oxidative phosphorylation, and cell cycle/apoptosis were shared between sexes. Among the sex-specific pathways were vitamins and cofactors metabolism and ion channel transport for females, and phospholipid, lysophospholipid, and phosphatidylinositol metabolism and insulin signaling for males. Additionally, numerous lipid and insulin-related pathways and inflammatory processes in the adipose and liver tissue appeared to show more prominent association with NAFLD in male HMDP. Using data-driven network modeling, we identified plausible sex-specific and tissue-specific regulatory genes as well as those that are shared between sexes. These key regulators orchestrate the NAFLD pathways in a sex- and tissue-specific manner. Gonadectomy experiments support that sex hormones may partially underlie the sexually dimorphic genes and pathways involved in NAFLD.
Conclusions: Our multi-omics integrative study reveals sex- and tissue-specific genes, processes, and networks underlying sexual dimorphism in NAFLD and may facilitate sex-specific precision medicine.
Keywords: Bayesian networks; Coexpression networks; Hybrid mouse diversity panel; Key regulator genes; Multi-omics integration; Non-alcoholic fatty liver disease (NAFLD); Sexual dimorphism.
Conflict of interest statement
Ethics approval and consent to participate
All data utilized in the current study were generated and published previously. Ethical issues have been addressed appropriately by the initial studies.
Consent for publication
Since our manuscript does not contain any data from any individual person, consent for publication is not applicable for this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- NMW d A, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48:S104–S112. - PubMed
-
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. 10.1002/hep.30113. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

