T-type Calcium Channels Determine the Vulnerability of Dopaminergic Neurons to Mitochondrial Stress in Familial Parkinson Disease
- PMID: 30344006
- PMCID: PMC6234903
- DOI: 10.1016/j.stemcr.2018.09.006
T-type Calcium Channels Determine the Vulnerability of Dopaminergic Neurons to Mitochondrial Stress in Familial Parkinson Disease
Abstract
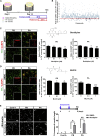
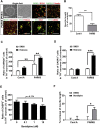
Parkinson disease (PD) is a progressive neurological disease caused by selective degeneration of dopaminergic (DA) neurons in the substantia nigra. Although most cases of PD are sporadic cases, familial PD provides a versatile research model for basic mechanistic insights into the pathogenesis of PD. In this study, we generated DA neurons from PARK2 patient-specific, isogenic PARK2 null and PARK6 patient-specific induced pluripotent stem cells and found that these neurons exhibited more apoptosis and greater susceptibility to rotenone-induced mitochondrial stress. From phenotypic screening with an FDA-approved drug library, one voltage-gated calcium channel antagonist, benidipine, was found to suppress rotenone-induced apoptosis. Furthermore, we demonstrated the dysregulation of calcium homeostasis and increased susceptibility to rotenone-induced stress in PD, which is prevented by T-type calcium channel knockdown or antagonists. These findings suggest that calcium homeostasis in DA neurons might be a useful target for developing new drugs for PD patients.
Keywords: PARK2; Parkinson disease; T-type calcium channels; disease modeling; induced pluripotent stem cells.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Athauda D., Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015;11:25–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

