Chd2 Is Necessary for Neural Circuit Development and Long-Term Memory
- PMID: 30344048
- PMCID: PMC6479120
- DOI: 10.1016/j.neuron.2018.09.049
Chd2 Is Necessary for Neural Circuit Development and Long-Term Memory
Abstract
Considerable evidence suggests loss-of-function mutations in the chromatin remodeler CHD2 contribute to a broad spectrum of human neurodevelopmental disorders. However, it is unknown how CHD2 mutations lead to impaired brain function. Here we report mice with heterozygous mutations in Chd2 exhibit deficits in neuron proliferation and a shift in neuronal excitability that included divergent changes in excitatory and inhibitory synaptic function. Further in vivo experiments show that Chd2+/- mice displayed aberrant cortical rhythmogenesis and severe deficits in long-term memory, consistent with phenotypes observed in humans. We identified broad, age-dependent transcriptional changes in Chd2+/- mice, including alterations in neurogenesis, synaptic transmission, and disease-related genes. Deficits in interneuron density and memory caused by Chd2+/- were reproduced by Chd2 mutation restricted to a subset of inhibitory neurons and corrected by interneuron transplantation. Our results provide initial insight into how Chd2 haploinsufficiency leads to aberrant cortical network function and impaired memory.
Keywords: MGE transplantation; autism; epigenetics; epilepsy; hippocampus; intellectual disability; interneuron; synaptic transmission.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures

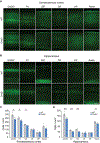




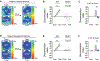
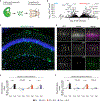
Comment in
-
CHD2: One Gene, Many Roles.Neuron. 2018 Dec 5;100(5):1014-1016. doi: 10.1016/j.neuron.2018.11.036. Neuron. 2018. PMID: 30521773
References
-
- Buckmaster PS (2012) Mossy fiber sprouting in the dentate gyrus. Jasper’s Basic Mechanisms of the Epilepsies 4th edn Eds: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (National Center for Biotechnology, Bethesda Maryland: ). - PubMed
-
- Carvill GL, Heavin SB, Yendle SC, McMahon JM, O’Roak BJ, Cook J, Khan A, Dorschner MO, Weaver M, Calvert S, Malone S, Wallace G, Stanley T, Bye AM, Bleasel A, Howell KB, Kivity S, Mackay MT, Rodriguez-Casero V, Webster R, Korczyn A, Afawi Z, Zelnick N, Lerman-Sagie T, Lev D, Møller RS, Gill D, Andrade DM, Freeman JL, S adleir LG, Shendure J, Berkovic SF, Scheffer IE, Mefford H (2013) Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 45, 825–830. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

