Therapeutic Efficacy and Safety of Prolonged Macrolide, Corticosteroid, Doxycycline, and Levofloxacin against Macrolide-Unresponsive Mycoplasma pneumoniae Pneumonia in Children
- PMID: 30344461
- PMCID: PMC6193889
- DOI: 10.3346/jkms.2018.33.e268
Therapeutic Efficacy and Safety of Prolonged Macrolide, Corticosteroid, Doxycycline, and Levofloxacin against Macrolide-Unresponsive Mycoplasma pneumoniae Pneumonia in Children
Abstract
Background: We aimed to compare the therapeutic efficacy of prolonged macrolide (PMC), corticosteroids (CST), doxycycline (DXC), and levofloxacin (LFX) against macrolide-unresponsive Mycoplasma pneumoniae (MP) pneumonia in children and to evaluate the safety of the secondary treatment agents.
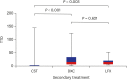
Methods: We retrospectively analyzed the data of patients with MP pneumonia hospitalized between January 2015 and April 2017. Macrolide-unresponsiveness was clinically defined with a persistent fever of ≥ 38.0°C at ≥ 72 hours after macrolide treatment. The cases were divided into four groups: PMC, CST, DXC, and LFX. We compared the time to defervescence (TTD) after secondary treatment and the TTD after initial macrolide treatment in each group with adjustment using propensity score-matching analysis.
Results: Among 1,165 cases of MP pneumonia, 190 (16.3%) were unresponsive to macrolides. The proportion of patients who achieved defervescence within 48 hours in CST, DXC, and LFX groups were 96.9% (31/33), 85.7% (12/14), and 83.3% (5/6), respectively. The TTD after initial macrolide treatment did not differ between PMC and CST groups (5.1 vs. 4.2 days, P = 0.085), PMC and DXC groups (4.9 vs. 5.7 days, P = 0.453), and PMC and LFX groups (4.4 vs. 5.0 days, P = 0.283). No side effects were observed in the CST, DXC, and LFX groups.
Conclusion: The change to secondary treatment did not show better efficacy compared to PMC in children with macrolide-unresponsive MP pneumonia. Further studies are needed to guide appropriate treatment in children with MP pneumonia.
Keywords: Antibiotics; Mycoplasma pneumoniae; Pneumonia.
Conflict of interest statement
Disclosure: The authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
Wanted: The Best Second Option to Treat Macrolide-Unresponsive Mycoplasmal Pneumonia in Children.J Korean Med Sci. 2018 Oct 10;33(43):e281. doi: 10.3346/jkms.2018.33.e281. eCollection 2018 Oct 22. J Korean Med Sci. 2018. PMID: 30344464 Free PMC article. No abstract available.
References
-
- Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16(3):162–169. - PubMed
-
- Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32(6):956–973. - PubMed
-
- Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008;56(5):326–331. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

