Regulatory NLRs Control the RLR-Mediated Type I Interferon and Inflammatory Responses in Human Dendritic Cells
- PMID: 30344524
- PMCID: PMC6182093
- DOI: 10.3389/fimmu.2018.02314
Regulatory NLRs Control the RLR-Mediated Type I Interferon and Inflammatory Responses in Human Dendritic Cells
Abstract
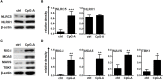
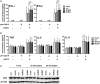
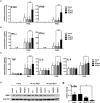
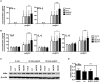
Unique members of the nucleotide-binding domain leucine-rich repeat (NLR) family have been found to regulate intracellular signaling pathways initiated by other families of pattern recognition receptors (PRR) such as Toll-like receptors (TLRs) and retinoic-acid inducible gene I (RIG-I)-like receptors (RLRs). Plasmacytoid dendritic cells (pDCs), the most powerful type I interferon (IFN) producing cells, preferentially employ endosomal TLRs to elicit antiviral IFN responses. By contrast, conventional DCs (cDCs) predominantly use cytosolic RLRs, which are constitutively expressed in them, to sense foreign nucleic acids. Previously we have reported that, though RIG-I is absent from resting pDCs, it is inducible upon TLR stimulation. In the recent study we investigated the regulatory ability of NLRs, namely NLRC5 and NLRX1 directly associated with the RLR-mediated signaling pathway in DC subtypes showing different RLR expression, particularly in pDCs, and monocyte-derived DCs (moDCs). Here we demonstrate that similarly to RLRs, NLRC5 is also inducible upon TLR9 stimulation, whereas NLRX1 is constitutively expressed in pDCs. Inhibition of NLRC5 and NLRX1 expression in pDCs augmented the RLR-stimulated expression of type I IFNs but did not affect the production of the pro-inflammatory cytokines TNF, IL-6, and the chemokine IL-8. Further we show that immature moDCs constantly express RLRs, NLRX1 and NLRC5 that are gradually upregulated during their differentiation. Similarly to pDCs, NLRX1 suppression increased the RLR-induced production of type I IFNs in moDCs. Interestingly, RLR stimulation of NLRX1-silenced moDCs leads to a significant increase in pro-inflammatory cytokine production and IκBα degradation, suggesting increased NF-κB activity. On the contrary, NLRC5 does not seem to have any effect on the RLR-mediated cytokine responses in moDCs. In summary, our results indicate that NLRX1 negatively regulates the RLR-mediated type I IFN production both in pDCs and moDCs. Further we show that NLRX1 inhibits pro-inflammatory cytokine secretion in moDCs but not in pDCs following RLR stimulation. Interestingly, NLRC5 suppresses the RLR-induced type I IFN secretion in pDCs but does not appear to have any regulatory function on the RLR pathway in moDCs. Collectively, our work demonstrates that RLR-mediated innate immune responses are primarily regulated by NLRX1 and partly controlled by NLRC5 in human DCs.
Keywords: NLR; RLR; antiviral; dendritic cell; interferon; regulate.
Figures






Similar articles
-
Regulation of RLR-Mediated Antiviral Responses of Human Dendritic Cells by mTOR.Front Immunol. 2020 Sep 11;11:572960. doi: 10.3389/fimmu.2020.572960. eCollection 2020. Front Immunol. 2020. PMID: 33013932 Free PMC article.
-
Human Plasmacytoid and Monocyte-Derived Dendritic Cells Display Distinct Metabolic Profile Upon RIG-I Activation.Front Immunol. 2018 Dec 21;9:3070. doi: 10.3389/fimmu.2018.03070. eCollection 2018. Front Immunol. 2018. PMID: 30622542 Free PMC article.
-
Regulation of type I interferon responses by mitochondria-derived reactive oxygen species in plasmacytoid dendritic cells.Redox Biol. 2017 Oct;13:633-645. doi: 10.1016/j.redox.2017.07.016. Epub 2017 Jul 29. Redox Biol. 2017. PMID: 28818792 Free PMC article.
-
Progresses on three pattern recognition receptor families (TLRs, RLRs and NLRs) in teleost.Dev Comp Immunol. 2021 Sep;122:104131. doi: 10.1016/j.dci.2021.104131. Epub 2021 May 19. Dev Comp Immunol. 2021. PMID: 34022258 Review.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
-
The lncRNAs involved in regulating the RIG-I signaling pathway.Front Cell Infect Microbiol. 2022 Nov 9;12:1041682. doi: 10.3389/fcimb.2022.1041682. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439216 Free PMC article. Review.
-
Regulation of RLR-Mediated Antiviral Responses of Human Dendritic Cells by mTOR.Front Immunol. 2020 Sep 11;11:572960. doi: 10.3389/fimmu.2020.572960. eCollection 2020. Front Immunol. 2020. PMID: 33013932 Free PMC article.
-
Anti-Cancer and Pro-Immune Effects of Lauric Acid on Colorectal Cancer Cells.Int J Mol Sci. 2025 Feb 24;26(5):1953. doi: 10.3390/ijms26051953. Int J Mol Sci. 2025. PMID: 40076581 Free PMC article.
-
Conceptus-modulated innate immune function during early pregnancy in ruminants: a review.Anim Reprod. 2021 May 10;18(1):e20200048. doi: 10.1590/1984-3143-AR2020-0048. Anim Reprod. 2021. PMID: 34122650 Free PMC article. Review.
-
Development and validation of an interferon signature predicting prognosis and treatment response for glioblastoma.Oncoimmunology. 2019 Jun 12;8(9):e1621677. doi: 10.1080/2162402X.2019.1621677. eCollection 2019. Oncoimmunology. 2019. PMID: 31428519 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

