The Gut-Microglia Connection: Implications for Central Nervous System Diseases
- PMID: 30344525
- PMCID: PMC6182051
- DOI: 10.3389/fimmu.2018.02325
The Gut-Microglia Connection: Implications for Central Nervous System Diseases
Abstract
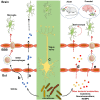
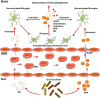
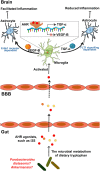
The importance of the gut microbiome in central nervous system (CNS) diseases has long been recognized; however, research into this connection is limited, in part, owing to a lack of convincing mechanisms because the brain is a distant target of the gut. Previous studies on the brain revealed that most of the CNS diseases affected by the gut microbiome are closely associated with microglial dysfunction. Microglia, the major CNS-resident macrophages, are crucial for the immune response of the CNS against infection and injury, as well as for brain development and function. However, the current understanding of the mechanisms controlling the maturation and function of microglia is obscure, especially regarding the extrinsic factors affecting microglial function during the developmental process. The gut microflora has been shown to significantly influence microglia from before birth until adulthood, and the metabolites generated by the microbiota regulate the inflammation response mediated by microglia in the CNS; this inspired our hypothesis that microglia act as a critical mediator between the gut microbiome and CNS diseases. Herein, we highlight and discuss current findings that show the influence of host microbiome, as a crucial extrinsic factor, on microglia within the CNS. In addition, we summarize the CNS diseases associated with both the host microbiome and microglia and explore the potential pathways by which the gut bacteria influence the pathogenesis of CNS diseases. Our work is thus a comprehensive theoretical foundation for studies on the gut-microglia connection in the development of CNS diseases; and provides great potential for researchers to target pathways associated with the gut-microglia connection and overcome CNS diseases.
Keywords: brain; central nervous system diseases; gut microbiome; gut-microglia connection; microglia.
Figures
Similar articles
-
The microbiota-microglia axis in central nervous system disorders.Brain Pathol. 2020 Nov;30(6):1159-1177. doi: 10.1111/bpa.12908. Epub 2020 Nov 5. Brain Pathol. 2020. PMID: 33073887 Free PMC article. Review.
-
Microbes, microglia, and pain.Neurobiol Pain. 2020 Jan 29;7:100045. doi: 10.1016/j.ynpai.2020.100045. eCollection 2020 Jan-Jul. Neurobiol Pain. 2020. PMID: 32072077 Free PMC article. Review.
-
Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases.Int J Mol Sci. 2019 Jun 25;20(12):3109. doi: 10.3390/ijms20123109. Int J Mol Sci. 2019. PMID: 31242699 Free PMC article. Review.
-
Microglia and Microbiome-Gut-Brain Axis.Adv Neurobiol. 2024;37:303-331. doi: 10.1007/978-3-031-55529-9_17. Adv Neurobiol. 2024. PMID: 39207699 Review.
-
Recent advances in the understanding of microglial development and homeostasis.Cell Immunol. 2018 Aug;330:68-78. doi: 10.1016/j.cellimm.2018.01.004. Epub 2018 Jan 10. Cell Immunol. 2018. PMID: 29366562 Review.
Cited by
-
Disentangling the Hypothesis of Host Dysosmia and SARS-CoV-2: The Bait Symptom That Hides Neglected Neurophysiological Routes.Front Physiol. 2020 Jun 5;11:671. doi: 10.3389/fphys.2020.00671. eCollection 2020. Front Physiol. 2020. PMID: 32581854 Free PMC article.
-
The function of gut microbiota in immune-related neurological disorders: a review.J Neuroinflammation. 2022 Jun 15;19(1):154. doi: 10.1186/s12974-022-02510-1. J Neuroinflammation. 2022. PMID: 35706008 Free PMC article. Review.
-
Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral.Molecules. 2019 Apr 17;24(8):1517. doi: 10.3390/molecules24081517. Molecules. 2019. PMID: 30999685 Free PMC article. Review.
-
Gut-Induced Inflammation during Development May Compromise the Blood-Brain Barrier and Predispose to Autism Spectrum Disorder.J Clin Med. 2020 Dec 24;10(1):27. doi: 10.3390/jcm10010027. J Clin Med. 2020. PMID: 33374296 Free PMC article. Review.
-
Gastrointestinal Dysfunction and Low-Grade Inflammation Associate With Enteric Neuronal Amyloid-β in a Model for Amyloid Pathology.Neurogastroenterol Motil. 2025 May;37(5):e15016. doi: 10.1111/nmo.15016. Epub 2025 Mar 6. Neurogastroenterol Motil. 2025. PMID: 40051115 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

