The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses
- PMID: 30344997
- PMCID: PMC6180374
- DOI: 10.1177/2045125318781475
The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses
Abstract
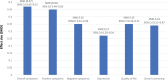
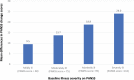
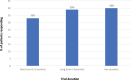
Schizophrenia is the eighth leading cause of disability worldwide in people aged 15-44 years. Before antidopaminergic antipsychotics were introduced in the 1950s, no effective medications existed for the treatment of schizophrenia. This review summarizes key meta-analytic findings regarding antipsychotic efficacy in the acute treatment of schizophrenia, including clozapine in treatment-resistant patients. In the most comprehensive meta-analysis of randomized controlled trials conducted in multi-episode schizophrenia, antipsychotics outperformed placebo regarding total symptoms, positive symptoms, negative symptoms, depressive symptoms, quality of life and social functioning. Amongst these outcomes, the standardized mean difference for overall symptoms was largest, that is, 0.47 (95% credible interval = 0.42-0.51), approaching a medium effect size, being reduced to 0.38 when publication bias and small-trial effects were accounted for. A comparison of two meta-analyses indicated that first-episode patients, compared with multi-episode patients, were more likely to have at least minimal treatment response [⩾20% Positive and Negative Syndrome Scale (PANSS)/Brief Psychiatric Rating Scale (BPRS) score reduction: 81% versus 51%] and good response (⩾50% PANSS/BPRS score reduction: 52% versus 23%). In multi-episode schizophrenia, no response or worsening after 2 weeks of a therapeutic antipsychotic dose was highly predictive of not achieving a good response at endpoint (median treatment = 6 weeks: specificity = 86%; positive predictive value = 90%), suggesting a change in treatment should be considered in such cases. In first-episode psychosis, adequately dosed antipsychotic treatment trials for more than 2 weeks are recommended before using no response or worsening as a decision point for aborting a given antipsychotic. In clearly defined treatment-resistant schizophrenia, clozapine generally outperformed other antipsychotics, especially when dosed appropriately (target = 3-6 months' duration; trough clozapine level ⩾350-400 μg/L) with a response rate (⩾20% PANSS/BPRS) of 33% by 3 months of treatment. High antipsychotic doses and psychotropic combinations are unlikely to be superior to standard doses of antipsychotic monotherapy. Acute antipsychotic efficacy in schizophrenia depends on the targeted symptom domain (greater efficacy: total and positive symptoms, lesser efficacy: negative symptoms, depressive symptoms, social functioning and quality of life). Greater antipsychotic efficacy is associated with higher total baseline symptom severity, treatment-naïveté/first-episode status, shorter illness duration, and trials that are nonindustry sponsored and that have a lower placebo effect. The heterogeneity of antipsychotic response across individuals and key symptom domains, the considerable degree of nonresponse/treatment resistance in multi-episode patients, and the adverse effect potential of antipsychotics are major limitations, underscoring the need to develop new medications for the treatment of schizophrenia. Drug development should include matching patient subgroups, which are identified by means of clinical and biomarker variables, to mechanisms of action of novel medications, targeting specific symptom domains, and investigating mechanisms of action other than dopaminergic blockade.
Keywords: antipsychotic; clozapine; efficacy; meta-analysis; randomized controlled trial; response; schizophrenia.
Conflict of interest statement
Conflict of interest statement: In the last 3 years, Dr Haddad has received honoraria for lecturing and consultancy work from Allergan, Galen, Janssen, Lundbeck, NewBridge Pharmaceuticals, Otsuka, Sunovion and Teva, plus conference support from Janssen, Lundbeck, NewBridge Pharmaceuticals and Sunovion. Dr Correll has been a consultant or advisor to or has received honoraria from Alkermes, Allergan, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, ROVI, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Pfizer, Roche and ROVI. He received royalties from UpToDate and grant support from Janssen, Neurocrine and Takeda. He is also a shareholder of LB Pharma.
Figures


References
-
- Peräla J, Suvisaari J, Samuli I, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 2007; 64: 19–28. - PubMed
-
- World Health Organisation. The WHO World Health Report 2001 - Mental Health: New Understanding, New Hope. Geneva: World Health Organization, 2001.
-
- Cloutier M, Aigbogun MS, Guerin A, et al. The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 2016; 77: 764–771. - PubMed
-
- López-Muñoz F, Alamo C, Cuenca E, et al. History of the discovery and clinical introduction of chlorpromazine. Ann Clin Psychiatry 2005; 17: 113–135. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

