Synthetic immunology: T-cell engineering and adoptive immunotherapy
- PMID: 30345403
- PMCID: PMC6190530
- DOI: 10.1016/j.synbio.2018.08.001
Synthetic immunology: T-cell engineering and adoptive immunotherapy
Erratum in
-
Erratum regarding previously published articles.Synth Syst Biotechnol. 2020 Oct 12;5(4):328. doi: 10.1016/j.synbio.2020.10.003. eCollection 2020 Dec. Synth Syst Biotechnol. 2020. PMID: 33102826 Free PMC article.
Abstract
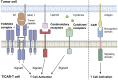
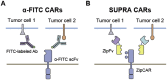
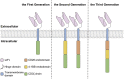
During the past decades, the rapidly-evolving cancer is hard to be thoroughly eliminated even though the radiotherapy and chemotherapy do exhibit efficacy in some degree. However, a breakthrough appeared when the adoptive cancer therapy [1] was developed, especially T cells armed with chimeric antigen receptors (CARs) showed great potential in tumor clinical trials recently. CAR-T cells successfully elevated the efficiency and specificity of cytotoxicity. In this review, we will talk about the design of CAR and CAR-included combinatory therapeutic applications in the principles of systems and synthetic immunology.
Keywords: Cell therapy; Chimeric antigen receptor (CAR); Immunotherapy; Systems and synthetic biology; Systems and synthetic immunology; T cell signaling; Tumor immunology.
Figures


References
-
- Kershaw M.H., Westwood J.A., Darcy P.K. Gene-engineered T cells for cancer therapy. Nat Rev Canc. 2013;13(8):525–541. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

