Comparison of the metabolism of 10 chemicals in human and pig skin explants
- PMID: 30345528
- PMCID: PMC6587507
- DOI: 10.1002/jat.3730
Comparison of the metabolism of 10 chemicals in human and pig skin explants
Abstract
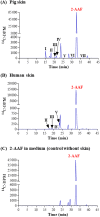
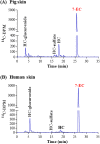
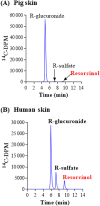
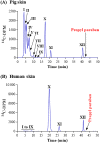
Skin metabolism is important to consider when assessing local toxicity and/or penetration of chemicals and their metabolites. If human skin supply is limited, pig skin can be used as an alternative. To identify any species differences, we have investigated the metabolism of 10 chemicals in a pig and human skin explant model. Phase I metabolic pathways in skin from both species included those known to occur via cytochrome P450s, esterases, alcohol dehydrogenases and aldehyde dehydrogenases. Common Phase II pathways were glucuronidation and sulfation but other conjugation pathways were also identified. Chemicals not metabolized by pig skin (caffeine, IQ and 4-chloroaniline) were also not metabolized by human skin. Six chemicals metabolized by pig skin were metabolized to a similar extent (percentage parent remaining) by human skin. Human skin metabolites were also detected in pig skin incubations, except for one unidentified minor vanillin metabolite. Three cinnamyl alcohol metabolites were unique to pig skin but represented minor metabolites. There were notable species differences in the relative amounts of common metabolites. The difference in the abundance of the sulfate conjugates of resorcinol and 4-amino-3-nitrophenol was in accordance with the known lack of aryl sulfotransferase activity in pigs. In conclusion, while qualitative comparisons of metabolic profiles were consistent between pig and human skin, there were some quantitative differences in the percentage of metabolites formed. This preliminary assessment suggests that pig skin is metabolically competent and could be a useful tool for evaluating potential first-pass metabolism before testing in human-derived tissues.
Keywords: comparison; cosmetics chemicals; human; pig; skin metabolism.
© 2018 The Authors Journal of Applied Toxicology Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures

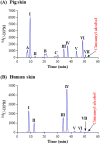
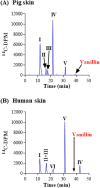
References
-
- Abbas, S. , Greige‐Gerges, H. , Karam, N. , Piet, M. H. , Netter, P. , & Magdalou, J. (2010). Metabolism of parabens (4‐hydroxybenzoic acid esters) by hepatic esterases and UDP‐glucuronosyltransferases in man. Drug Metabolism and Pharmacokinetics, 25, 568–577. - PubMed
-
- Berthout, F. , Ratanasavanh, D. , Riche, C. , Picart, D. , Voirin, T. , & Guillouzo, A. (1989). Comparison of caffeine metabolism by slices, microsomes and hepatocyte culture from adult human liver. Xenobiotica, 19, 401–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

