A Quantitative Model for BicD2/Cargo Interactions
- PMID: 30345745
- PMCID: PMC6520106
- DOI: 10.1021/acs.biochem.8b00987
A Quantitative Model for BicD2/Cargo Interactions
Abstract
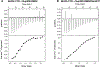
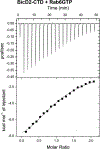
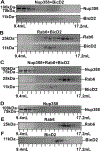
Dynein adaptor proteins such as Bicaudal D2 (BicD2) are integral components of the dynein transport machinery, as they recognize cargoes for cell cycle-specific transport and link them to the motor complex. Human BicD2 switches from selecting secretory and Golgi-derived vesicles for transport in G1 and S phase (by recognizing Rab6GTP), to selecting the nucleus for transport in G2 phase (by recognizing nuclear pore protein Nup358), but the molecular mechanisms governing this switch are elusive. Here, we have developed a quantitative model for BicD2/cargo interactions that integrates affinities, oligomeric states, and cellular concentrations of the reactants. BicD2 and cargo form predominantly 2:2 complexes. Furthermore, the affinity of BicD2 toward its cargo Nup358 is higher than that toward Rab6GTP. Based on our calculations, an estimated 1000 BicD2 molecules per cell would be recruited to the nucleus through Nup358 in the absence of regulation. Notably, RanGTP is a negative regulator of the Nup358/BicD2 interaction that weakens the affinity by a factor of 10 and may play a role in averting dynein recruitment to the nucleus outside of the G2 phase. However, our quantitative model predicts that an additional negative regulator remains to be identified. In the absence of negative regulation, the affinity of Nup358 would likely be sufficient to recruit BicD2 to the nucleus in G2 phase. Our quantitative model makes testable predictions of how cellular transport events are orchestrated. These transport processes are important for brain development, cell cycle control, signaling, and neurotransmission at synapses.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Splinter D, Razafsky DS, Schlager MA, Serra-Marques A, Grigoriev I, Demmers J, Keijzer N, Jiang K, Poser I, Hyman AA, Hoogenraad CC, King SJ, and Akhmanova A (2012) BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol. Biol. Cell 23, 4226–4241. - PMC - PubMed
-
- Schlager MA, Serra-Marques A, Grigoriev I, Gumy LF, Esteves da Silva M, Wulf PS, Akhmanova A, and Hoogenraad CC (2014) Bicaudal D family adaptor proteins control the velocity of dynein-based movements. Cell Rep 8, 1248–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

