Skin preparation for preventing infection following caesarean section
- PMID: 30346040
- PMCID: PMC6517158
- DOI: 10.1002/14651858.CD007462.pub4
Skin preparation for preventing infection following caesarean section
Update in
-
Skin preparation for preventing infection following caesarean section.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD007462. doi: 10.1002/14651858.CD007462.pub5. Cochrane Database Syst Rev. 2020. PMID: 32580252 Free PMC article.
Abstract
Background: The risk of maternal mortality and morbidity (particularly postoperative infection) is higher for caesarean section (CS) than for vaginal birth. With the increasing rate of CS, it is important to minimise the risks to the mother as much as possible. This review focused on different forms and methods of preoperative skin preparation to prevent infection. This review is an update of a review that was first published in 2012, and updated in 2014.
Objectives: To compare the effects of different antiseptic agents, different methods of application, or different forms of antiseptic used for preoperative skin preparation for preventing postcaesarean infection.
Search methods: For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (27 November 2017), and reference lists of retrieved studies.
Selection criteria: Randomised and quasi-randomised trials, evaluating any type of preoperative skin preparation agents, forms, and methods of application for caesarean section.Comparisons of interest in this review were between different antiseptic agents used for CS skin preparation (e.g. alcohol, povidone iodine), different methods of antiseptic application (e.g. scrub, paint, drape), different forms of antiseptic (e.g. powder, liquid), and also between different skin preparations, such as a plastic incisional drape, which may or may not be impregnated with antiseptic agents.Only studies involving the preparation of the incision area were included. This review did not cover studies of preoperative handwashing by the surgical team or preoperative bathing.
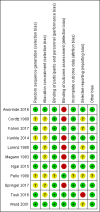
Data collection and analysis: Three review authors independently assessed all potential studies for inclusion, assessed risk of bias, and extracted the data using a predesigned form. We checked data for accuracy. We assessed the quality of the evidence using the GRADE approach.
Main results: For this update, we included 11 randomised controlled trials (RCTs), with a total of 6237 women who were undergoing CS. Ten trials (6215 women) contributed data to this review. All included studies were individual RCTs. We did not identify any quasi- or cluster-RCTs. The trial dates ranged from 1983 to 2016. Six trials were conducted in the USA, and the remainder in Nigeria, South Africa, France, Denmark, and Indonesia.The included studies were broadly methodologically sound, but raised some specific concerns regarding risk of bias in a number of cases.Drape versus no drapeThis comparison investigated the use of a non-impregnated drape versus no drape, following preparation of the skin with antiseptics. For women undergoing CS, low-quality evidence suggested that using a drape before surgery compared with no drape, may make little or no difference to the incidence of surgical site infection (risk ratio (RR) 1.29, 95% confidence interval (CI) 0.97 to 1.71; 2 trials, 1294 women), or length of stay in the hospital (mean difference (MD) 0.10 day, 95% CI -0.27 to 0.46 1 trial, 603 women).One-minute alcohol scrub with iodophor drape versus five-minute iodophor scrub without drapeOne trial compared an alcohol scrub and iodophor drape with a five-minute iodophor scrub only, and reported no surgical site infection in either group (79 women, very-low quality evidence). We were uncertain whether the combination of a one-minute alcohol scrub and a drape reduced the incidence of endomyometritis when compared with a five-minute scrub, because the quality of the evidence was very low (RR 1.62, 95% CI 0.29 to 9.16; 1 trial, 79 women).Parachlorometaxylenol with iodine versus iodine aloneWe were uncertain whether parachlorometaxylenol with iodine before CS made any difference to the incidence of surgical site infection (RR 0.33, 95% CI 0.04 to 2.99; 1 trial, 50 women), or endometritis (RR 0.88, 95% CI 0.56 to 1.38; 1 trial, 50 women) when compared with iodine alone, because the quality of the evidence was very low.Chlorhexidine gluconate versus povidone iodineLow-quality evidence suggested that chlorhexidine gluconate before CS, when compared with povidone iodine, may make little or no difference to the incidence of surgical site infection (RR 0.80, 95% CI 0.62 to 1.02; 6 trials, 3607 women). However, surgical site infection appeared to be slightly reduced for women for whom chlorhexidine gluconate was used compared with povidone iodine after we removed four trials at high risk of bias for outcome assessment, in a sensitivity analysis (RR 0.59, 95% CI 0.37 to 0.95; 2 trials, 1321 women).Low-quality evidence indicated that chlorhexidine gluconate before CS, when compared with povidone iodine, may make little or no difference to the incidence of endometritis (RR 1.01, 95% CI 0.51 to 2.01; 2 trials, 2079 women), or to reducing maternal skin irritation or allergic skin reaction (RR 0.60, 95% CI 0.22 to 1.63; 2 trials, 1521 women).One small study (60 women) reported reduced bacterial growth at 18 hours after CS for women who had chlorhexidine gluconate preparation compared with women who had povidone iodine preparation (RR 0.23, 95% CI 0.07 to 0.70).None of the included trials reported on maternal mortality or repeat surgery.Chlorhexidine 0.5% versus 70% alcohol plus drapeOne trial, which was only available as an abstract, investigated the effect of skin preparation on neonatal adverse events, and found cord blood iodine concentration to be higher in the iodine group.
Authors' conclusions: There was insufficient evidence available from the included RCTs to fully evaluate different agents and methods of skin preparation for preventing infection following caesarean section. Therefore, it is not yet clear what sort of skin preparation may be most effective for preventing postcaesarean surgical site infection, or for reducing other undesirable outcomes for mother and baby.Most of the evidence in this review was deemed to be very low or low quality. This means that for most findings, our confidence in any evidence of an intervention effect is limited, and indicates the need for more high-quality research.This field needs high quality, well designed RCTs, with larger sample sizes. High priority questions include comparing types of antiseptic (especially iodine versus chlorhexidine), and application methods (scrubbing, swabbing, or draping). We found four studies that were ongoing; we will incorporate the results of these studies in future updates of this review.
Conflict of interest statement
Diah R Hadiati: Diah Hadiati is a named author on Fahmi 2017, but was not involved in the screening process and risk of bias assessment.
Mohammad Hakimi: none known
Detty S Nurdiati: none known
Erika Ota: none known
Katharina da Silva Lopes: none known
Figures
Update of
-
Skin preparation for preventing infection following caesarean section.Cochrane Database Syst Rev. 2014 Sep 17;(9):CD007462. doi: 10.1002/14651858.CD007462.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Oct 22;10:CD007462. doi: 10.1002/14651858.CD007462.pub4. PMID: 25229700 Updated.
References
References to studies included in this review
Aworinde 2016 {published data only}
-
- Aworinde O, Olufemi‐Aworinde K, Fehintola A, Adeyemi B, Owonikoko M, Adeyemi AS. Antiseptic skin preparation for preventing surgical site infection at caesarean section. Open Journal of Obstetrics and Gynecology 2016;6:246‐51.
-
- PACTR201401000697382. Comparative study of antiseptic skin preparation for preventing surgical site infection at caesarean section: a randomised control trial. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201401... (first received 5 November 2013).
Cordtz 1989 {published data only}
-
- Cordtz T, Schouenborg L, Laursen K, Daugaard HO, Buur K, Munk Christensen B, et al. The effect of incisional plastic drapes and redisinfection of operation site on wound infection following caesarean section. Journal of Hospital Infection 1989;13(3):267‐72. [PUBMED: 2567756] - PubMed
Fahmi 2017 {published data only}
-
- Fahmi MN, Hadiati DR, Widad S. Comparison of skin preparation with alcohol‐chlorhexidine versus alcohol‐povidone iodine on surgical site infection following caesarean section. Journal of Obstetrics and Gynaecology Research 2017;43:38.
Kunkle 2014 {published data only}
-
- Murray C, Marchan J, Safadi S, Opper N, Yedigarova L, Chmait R. Efficacy of chlorhexidine gluconate versus povidone iodine for skin disinfection at cesarean section: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S152‐3.
-
- NCT01975805. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01975805. (first received 5 November 2013).
Lorenz 1988 {published data only}
-
- Lorenz RP, Botti JJ, Appelbaum PC, Bennett N. Skin preparation methods before cesarean section. Journal of Reproductive Medicine 1988;33(2):202‐4. - PubMed
Magann 1993 {published data only}
-
- Magann EF, Dodson MK, Ray MA, Harris RL, Martin JN, Morrison JC. Preoperative skin preparation and intraoperative pelvic irrigation: impact on endometritis following Cesarean delivery. 41st Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1993 May 3‐6; Washington DC, USA. 1993:11.
-
- Magann EF, Dodson MK, Ray MA, Harris RL, Martin JN, Morrison JC. Preoperative skin preparation and intraoperative pelvic irrigation: impact on post‐cesarean endometritis and wound infection. Obstetrics and Gynecology 1993;81(6):922‐5. [PUBMED: 8497357] - PubMed
Ngai 2015 {published data only}
-
- Maiwald M. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2017;129(4):750‐1. - PubMed
-
- Ngai I, Govindappagari S, Arsdale A, Judge NE, Neto N, Bernstein J, et al. Skin preparation in cesarean birth for prevention of surgical site infection (SSI): a prospective randomized clinical trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S424. - PubMed
-
- Ngai IM, Arsdale A, Govindappagari S, Judge NE, Neto NK, Bernstein J, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2015;126(6):1251‐7. - PubMed
Pello 1990 {published data only}
-
- Pello JY, Pons G, Leger FA, Moisson‐Tardieu MT, Dailhe‐Dupont D, Francoual C, et al. Ioban 2 for cesarean section operative field: study of innocuity for the newborn. Therapie 1990;45:85.
Springel 2017 {published data only}
-
- NCT02202577. Chlorhexidine‐alcohol versus povidone‐iodine for surgical site antisepsis prior to cesarean delivery. clinicaltrials.gov/ct2/show/NCT02202577 (first received 29 July 2014).
-
- Springel EH, Sarfoh V, Stetzer B, Weight S, Mercer B, Wang XY. A randomized controlled trial of chlorhexidine‐alcohol versus povidone‐iodine for cesarean antisepsis. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S30, Abstract no: 42. [NCT02202577] - PubMed
-
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open‐label controlled trial of chlorhexidine‐alcohol versus povidone‐iodine for cesarean antisepsis: the CAPICA trial. American Journal of Obstetrics and Gynecology 2017;217:463.e1‐8. - PubMed
Tuuli 2016 {published data only}
-
- NCT01472549. Antiseptic skin preparation for preventing surgical site infection at cesarean delivery: a randomized comparative effectiveness trial. clinicaltrials.gov/show/NCT01472549 (first received November 2011).
-
- Stout MJ, Martin S, Cahill AG, Macones GA, Tuuli MG. Impact of chlorhexidine‐alcohol versus iodine‐alcohol skin antisepsis on methicillin‐resistant staphylococcus aureus infection after cesarean. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S119, Abstract no: 194. [NCT01472549]
-
- Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Colditz G, et al. Chlorhexidine‐alcohol compared with iodine‐alcohol for preventing surgical‐site infection at cesarean: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S3, Abstract no: 4.
Ward 2001 {published data only}
-
- Ward HRG, Jennings OGN, Potgieter P, Lombard CJ. Do adhesive plastic drapes prevent post caesarean wound infection?. Journal of Hospital Infection 2001;47:230‐4. - PubMed
References to studies excluded from this review
Brown 1984 {published data only}
-
- Brown TR, Ehrlich CE, Stehman FB, Golichowski AM, Madura JA, Eitzen HE. A clinical evaluation of chlorhexidine gluconate spray as compared with iodophor scrub for preoperative skin preparation. Surgery, Gynecology and Obstetrics 1984;158(4):363‐6. - PubMed
Kosus 2010 {published data only}
-
- Kosus A, Kosus N, Guler A, Capar M. Rifamycin SV application to subcutaneous tissue for prevention of post‐cesarean surgical site infection [Sezaryen sonrasi kesi yeri enfeksiyonunu onlemek icin ciltalti rifamisin SV uygulanmasi]. European Journal of General Medicine 2010;7(3):269‐76.
NCT01700803 {published data only}
-
- NCT01700803. Povidone iodine and cesarean section wound infections. clinicaltrials.gov/ct2/show/NCT01700803 (first received 4 October 2012).
NCT02027324 {published data only}
-
- NCT02027324. Prevention of surgical site infection after cesarean delivery (CAPISSI). clinicaltrials.gov/ct2/show/NCT02027324 (first received 6 Janury 2014).
Nili 2015 {published data only}
-
- IRCT201204289568N1. Studying the relationship between neonatal hypothyroidism and using iodine and non iodine containing disinfectants before caesarian section in icteric neonates that refer to clinic of neonates in Valiasr hospital. en.irct.ir/trial/10126 (first registered 07 July 2012).
-
- Nili F, Hantoushzadeh S, Alimohamadi A, Shariat M, Rezaeizadeh G. Iodine‐containing disinfectants in preparation for caesarean section: impact on thyroid profile in cord blood. Postgraduate Medical Journal 2015;91:681‐84. - PubMed
Robins 2005 {published data only}
-
- Robins K, Wilson R, Watkins EJ, Columb MO, Lyons G. Chlorhexidine spray versus single use sachets for skin preparation before regional nerve blockade for elective caesarean section: an effectiveness, time and cost study. International Journal of Obstetric Anesthesia 2005;14(3):189‐92. - PubMed
References to ongoing studies
NCT00528008 {published data only}
-
- NCT00528008. A comparison of surgical preparations and wound infection rates for elective cesarean sections. clinicaltrials.gov/ct2/show/NCT00528008 (first received 11 September 2007).
NCT01870583 {published data only}
-
- NCT01870583. Comparison of surgical skin preps during cesarean deliveries. clinicaltrials.gov/ct2/show/record/NCT01870583 (first received 6 June 2013).
NCT02396329 {published data only}
-
- NCT02396329. Chlorhexidine versus povidone‐iodine antisepsis for reduction of post cesarean section surgical site infection rate:a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02396329 (first received 1 March 2015).
NCT02402907 {published data only}
-
- NCT02402907. A randomized trial to determine if a pre‐operative wash with a chlorhexidine cloth reduces infectious morbidity in patients undergoing cesarean section. clinicaltrials.gov/ct2/show/NCT02402907 (first received 30 March 2015).
Additional references
AORN 2002
-
- Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN Journal 2002;75:184‐7. - PubMed
CDC 2005
-
- Centers for Disease Control and Prevention. Data and statistics for surgical site infections. www.cdc.gov/ncidod/dhqp/dpac_ssi_data.html (accessed: 5 August 2008).
Cunningham 2018
-
- Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, et al. Puerperal complications. In: Hoffman BL editor(s). Williams Obstetrics. 25th Edition. McGraw‐Hill Education, 2018.
Dumville 2015
GRADE Handbook
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013.
GRADE Working Group 2004
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 26 September 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haas 2018
Hardin 1997
-
- Hardin W, Nichols R. Handwashing and patient skin preparation. In: Malangoni MA editor(s). Critical Issues in Operating Room Management. Philadelphia: Lippincott‐Raven, 1997:133‐44.
Henderson 1995
-
- Henderson E, Love EJ. Incidence of hospital‐acquired infections associated with caesarean section. Journal of Hospital Infection 1995;29:245‐55. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Larson 1988
-
- Larson E. Guideline for use of topical antimicrobial agents. American Journal of Infection Control 1988;16:253‐66. - PubMed
Leaper 2001
-
- Leaper DJ, Orr C, Maung Z, White A. Inflammation and Infection: STEP 2000 Module II.. Royal College of Surgeons of England: Blackwell Science, 2001.
Leclair 1990
-
- Leclair J. A review of antiseptics. Cleansing agents. Todays OR Nurse 1990;12(10):25‐8. - PubMed
Lewis 2013
Loudon 2002
-
- Loudon I. Ignaz Phillip Semmelweis' studies of death in childbirth. www.jameslindlibrary.org (accessed: 5 August 2008). - PMC - PubMed
Mangram 1999
-
- Mangram JA, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection. Infection Control and Hospital Epidemiology 1999;20:247‐78. - PubMed
Martin 2015
-
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: Final Data for 2015. National Vital Statistics Report 2017;66(1):1. [PUBMED: 28135188] - PubMed
Mathai 2013
NCT01472549
-
- NCT01472549. Antiseptic skin preparation for preventing surgical site infection at cesarean delivery: a randomized comparative effectiveness trial. clinicaltrials.gov/show/NCT01472549 (first received November 2011).
Normand 2001
-
- Normand MC, Damato EG. Postcesarean infection. JOGNN ‐ Journal of Obstetric, Gynecologic, & Neonatal Nursing 2001;30(6):642‐8. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2009
-
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] - PubMed
Sterne 2017
-
- Sterne JAC, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Thomas 2001
-
- Thomas J, Paranjothy S, Royal College of Obstetricians and Gynaecologists Clinical Effectiveness Support Unit. National Sentinel Caesarean Section Audit Report. London: RCOG Press, 2001.
Vogel 2015
-
- Vogel JP, Betran AP, Vindevoghel N, Souza JP, Torloni MR, Zhang J, et al. Use of the Robson classification to assess caesarean section trends in 21 countries: a secondary analysis of two WHO multicountry surveys. Lancet Global health 2015;3(5):e260‐70. [DOI: 10.1016/S2214-109X(15)70094-X; PUBMED: 25866355] - DOI - PubMed
WHO 2015
-
- World Health Organization. WHO Statement on Caesarean Section Rates. www.who.int/reproductivehealth/publications/maternal_perinatal_health/cs... 2015.
References to other published versions of this review
Hadiati 2008
Hadiati 2012
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical