Rapidly progressive amyotrophic lateral sclerosis is associated with microglial reactivity and small heat shock protein expression in reactive astrocytes
- PMID: 30346063
- PMCID: PMC7379307
- DOI: 10.1111/nan.12525
Rapidly progressive amyotrophic lateral sclerosis is associated with microglial reactivity and small heat shock protein expression in reactive astrocytes
Abstract
Aims: Amyotrophic lateral sclerosis (ALS) is a chronic neurodegenerative disease characterized by progressive loss of motor neurons, muscle weakness, spasticity, paralysis and death usually within 2-5 years of onset. Neuroinflammation is a hallmark of ALS pathology characterized by activation of glial cells, which respond by upregulating small heat shock proteins (HSPBs), but the exact underlying pathological mechanisms are still largely unknown. Here, we investigated the association between ALS disease duration, lower motor neuron loss, TARDNA-binding protein 43 (TDP-43) pathology, neuroinflammation and HSPB expression.
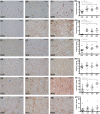
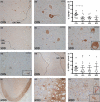
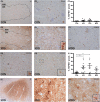
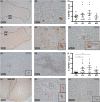
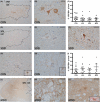
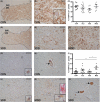
Methods: With immunohistochemistry, we examined HSPB1, HSPB5, HSPB6, HSPB8 and HSP16.2 expression in cervical, thoracic and sacral spinal cord regions in 12 ALS cases, seven with short disease duration (SDD), five with moderate disease duration (MDD), and ten age-matched controls. Expression was quantified using ImageJ to examine HSP expression, motor neuron numbers, microglial and astrocyte density and phosphorylated TDP-43 (pTDP-43+) inclusions.
Results: SDD was associated with elevated HSPB5 and 8 expression in lateral tract astrocytes, while HSP16.2 expression was increased in astrocytes in MDD cases. SDD cases had higher numbers of motor neurons and microglial activation than MDD cases, but similar levels of motor neurons with pTDP-43+ inclusions.
Conclusions: Increased expression of several HSPBs in lateral column astrocytes suggests that astrocytes play a role in the pathogenesis of ALS. SDD is associated with increased microgliosis, HSPB5 and 8 expression in astrocytes, and only minor changes in motor neuron loss. This suggests that the interaction between motor neurons, microglia and astrocytes determines neuronal fate and functional decline in ALS.
Keywords: HSPB; TDP-43 pathology; amyotrophic lateral sclerosis; astrocytes; inflammation; small heat shock proteins.
© 2018 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Figures

Similar articles
-
Heat shock proteins are differentially expressed in brain and spinal cord: implications for multiple sclerosis.Clin Exp Immunol. 2018 Nov;194(2):137-152. doi: 10.1111/cei.13186. Epub 2018 Sep 19. Clin Exp Immunol. 2018. PMID: 30014472 Free PMC article.
-
DREAM-Dependent Activation of Astrocytes in Amyotrophic Lateral Sclerosis.Mol Neurobiol. 2018 Jan;55(1):1-12. doi: 10.1007/s12035-017-0713-1. Mol Neurobiol. 2018. PMID: 28840473
-
Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis.Brain. 2020 Feb 1;143(2):430-440. doi: 10.1093/brain/awz419. Brain. 2020. PMID: 32040555 Free PMC article.
-
Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis.Curr Pharm Des. 2017;23(33):5010-5021. doi: 10.2174/1381612823666170622095802. Curr Pharm Des. 2017. PMID: 28641533 Free PMC article. Review.
-
Implications for soluble iron accumulation, oxidative stress, and glial glutamate release in motor neuron death associated with sporadic amyotrophic lateral sclerosis.Neuropathology. 2025 Jun;45(3):177-201. doi: 10.1111/neup.13033. Epub 2025 Mar 10. Neuropathology. 2025. PMID: 40065552 Free PMC article. Review.
Cited by
-
Machine learning hypothesis-generation for patient stratification and target discovery in rare disease: our experience with Open Science in ALS.Front Comput Neurosci. 2024 Jan 4;17:1199736. doi: 10.3389/fncom.2023.1199736. eCollection 2023. Front Comput Neurosci. 2024. PMID: 38260713 Free PMC article.
-
Insights on Human Small Heat Shock Proteins and Their Alterations in Diseases.Front Mol Biosci. 2022 Feb 25;9:842149. doi: 10.3389/fmolb.2022.842149. eCollection 2022. Front Mol Biosci. 2022. PMID: 35281256 Free PMC article. Review.
-
Small Heat Shock Proteins: Protein Aggregation Amelioration and Neuro- and Age-Protective Roles.Int J Mol Sci. 2025 Feb 11;26(4):1525. doi: 10.3390/ijms26041525. Int J Mol Sci. 2025. PMID: 40003991 Free PMC article. Review.
-
Metabolic Regulation of Glial Phenotypes: Implications in Neuron-Glia Interactions and Neurological Disorders.Front Cell Neurosci. 2020 Feb 11;14:20. doi: 10.3389/fncel.2020.00020. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32116564 Free PMC article. Review.
-
Regulation of physiological and pathological condensates by molecular chaperones.FEBS J. 2025 Jul;292(13):3271-3297. doi: 10.1111/febs.17390. Epub 2025 Jan 5. FEBS J. 2025. PMID: 39756021 Free PMC article. Review.
References
-
- van Es MA, Hardiman O, Chio A, Al‐Chalabi A, Pasterkamp RJ, Veldink JH, et al Amyotrophic lateral sclerosis. Lancet 2017; 390: 2084–98 - PubMed
-
- Berlowitz DJ, Howard ME, Fiore JFJ, Vander Hoorn S, O'Donoghue FJ, Westlake J, et al Identifying who will benefit from non‐invasive ventilation in amyotrophic lateral sclerosis/motor neurone disease in a clinical cohort. J Neurol Neurosurg Psychiatry 2016; 87: 280–6 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

