Suprachiasmatic function in a circadian period mutant: Duper alters light-induced activation of vasoactive intestinal peptide cells and PERIOD1 immunostaining
- PMID: 30346078
- PMCID: PMC6290923
- DOI: 10.1111/ejn.14214
Suprachiasmatic function in a circadian period mutant: Duper alters light-induced activation of vasoactive intestinal peptide cells and PERIOD1 immunostaining
Abstract
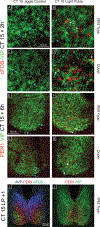
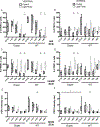
Mammalian circadian rhythms are entrained by photic stimuli that are relayed by retinal projections to the core of the suprachiasmatic nucleus (SCN). Neuronal activation, as demonstrated by expression of the immediate early gene c-fos, leads to transcription of the core clock gene per1. The duper mutation in hamsters shortens circadian period and amplifies light-induced phase shifts. We performed two experiments to compare the number of c-FOS immunoreactive (ir) and PER1-ir cells, and the intensity of staining, in the SCN of wild-type (WT) and duper hamsters at various intervals after presentation of a 15-min light pulse in the early subjective night. Light-induced c-FOS-ir within 1 hr in the dorsocaudal SCN of duper, but not WT hamsters. In cells that express vasoactive intestinal peptide (VIP), which plays a critical role in synchronization of SCN cellular oscillators, light-induced c-FOS-ir was greater in duper than WT hamsters. After the light pulse, PER1-ir cells were found in more medial portions of the SCN than FOS-ir, and appeared with a longer latency and over a longer time course, in VIP cells of duper than wild-type hamsters. Our results indicate that the duper allele alters SCN function in ways that may contribute to changes in free running period and phase resetting.
Keywords: PER1; VIP,; c-FOS; circadian mutant; duper.
© 2018 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
Competing Interests
The authors have no competing interests to declare.
Figures



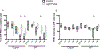
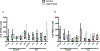
Similar articles
-
Phase resetting in duper hamsters: specificity to photic zeitgebers and circadian phase.J Biol Rhythms. 2015 Apr;30(2):129-43. doi: 10.1177/0748730414568297. Epub 2015 Jan 29. J Biol Rhythms. 2015. PMID: 25633984 Free PMC article.
-
Effects of the duper mutation on circadian responses to light.J Biol Rhythms. 2011 Aug;26(4):293-304. doi: 10.1177/0748730411411570. J Biol Rhythms. 2011. PMID: 21775288
-
Light-induced c-Fos expression in suprachiasmatic nuclei neurons targeting the paraventricular nucleus of the hamster hypothalamus: phase dependence and immunochemical identification.J Comp Neurol. 2002 Jan 1;442(1):48-62. doi: 10.1002/cne.1421. J Comp Neurol. 2002. PMID: 11754366
-
Circadian regulation of c-fos expression in the suprachiasmatic pacemaker by light.J Biol Rhythms. 1993;8 Suppl:S65-71. J Biol Rhythms. 1993. PMID: 8274764 Review.
-
An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review.
Cited by
-
Neuroendocrine effects of the duper mutation in Syrian hamsters: a role for Cryptochrome 1.Front Physiol. 2024 Feb 20;15:1351682. doi: 10.3389/fphys.2024.1351682. eCollection 2024. Front Physiol. 2024. PMID: 38444761 Free PMC article.
-
duper is a null mutation of Cryptochrome 1 in Syrian hamsters.Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2123560119. doi: 10.1073/pnas.2123560119. Epub 2022 Apr 26. Proc Natl Acad Sci U S A. 2022. PMID: 35471909 Free PMC article.
-
Anatomical Methods to Study the Suprachiasmatic Nucleus.Methods Mol Biol. 2022;2482:191-210. doi: 10.1007/978-1-0716-2249-0_13. Methods Mol Biol. 2022. PMID: 35610428 Free PMC article.
-
The duper mutation reveals previously unsuspected functions of Cryptochrome 1 in circadian entrainment and heart disease.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2121883119. doi: 10.1073/pnas.2121883119. Epub 2022 Aug 5. Proc Natl Acad Sci U S A. 2022. PMID: 35930669 Free PMC article.
References
-
- Aioun J, Chambille I, Peytevin J & Martinet L (1998). Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tiss Res., 291, 239–253. - PubMed
-
- Akiyama M, Kouzu Y, Takahahsi S, Wakamatsu H, Moriua T, Maetani M, Watanabe S, Tei H, Sakaki Y, & Shibata S (1999) Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci., 19, 1115–21. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

