Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells
- PMID: 30346102
- PMCID: PMC6351832
- DOI: 10.1111/acel.12841
Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells
Erratum in
-
Corrigendum.Aging Cell. 2019 Apr;18(2):e12942. doi: 10.1111/acel.12942. Aging Cell. 2019. PMID: 30860671 Free PMC article. No abstract available.
Abstract
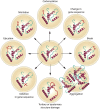
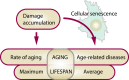
Understanding the aging process and ways to manipulate it is of major importance for biology and medicine. Among the many aging theories advanced over the years, the concept most consistent with experimental evidence posits the buildup of numerous forms of molecular damage as a foundation of the aging process. Here, we discuss that this concept integrates well with recent findings on cellular senescence, offering a novel view on the role of senescence in aging and age-related disease. Cellular senescence has a well-established role in cellular aging, but its impact on the rate of organismal aging is less defined. One of the most prominent features of cellular senescence is its association with macromolecular damage. The relationship between cell senescence and damage concerns both damage as a molecular signal of senescence induction and accelerated accumulation of damage in senescent cells. We describe the origin, regulatory mechanisms, and relevance of various damage forms in senescent cells. This view on senescent cells as carriers and inducers of damage puts new light on senescence, considering it as a significant contributor to the rise in organismal damage. Applying these ideas, we critically examine current evidence for a role of cellular senescence in aging and age-related diseases. We also discuss the differential impact of longevity interventions on senescence burden and other types of age-related damage. Finally, we propose a model on the role of aging-related damage accumulation and the rate of aging observed upon senescent cell clearance.
Keywords: aging; cellular senescence; deleteriome; evolutionary biology; lifespan; molecular damage; theories of aging.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

