Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness
- PMID: 30346242
- PMCID: PMC6364999
- DOI: 10.1056/NEJMoa1808217
Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness
Abstract
Background: There are conflicting data on the effects of antipsychotic medications on delirium in patients in the intensive care unit (ICU).
Methods: In a randomized, double-blind, placebo-controlled trial, we assigned patients with acute respiratory failure or shock and hypoactive or hyperactive delirium to receive intravenous boluses of haloperidol (maximum dose, 20 mg daily), ziprasidone (maximum dose, 40 mg daily), or placebo. The volume and dose of a trial drug or placebo was halved or doubled at 12-hour intervals on the basis of the presence or absence of delirium, as detected with the use of the Confusion Assessment Method for the ICU, and of side effects of the intervention. The primary end point was the number of days alive without delirium or coma during the 14-day intervention period. Secondary end points included 30-day and 90-day survival, time to freedom from mechanical ventilation, and time to ICU and hospital discharge. Safety end points included extrapyramidal symptoms and excessive sedation.
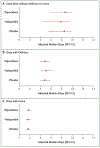
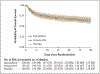
Results: Written informed consent was obtained from 1183 patients or their authorized representatives. Delirium developed in 566 patients (48%), of whom 89% had hypoactive delirium and 11% had hyperactive delirium. Of the 566 patients, 184 were randomly assigned to receive placebo, 192 to receive haloperidol, and 190 to receive ziprasidone. The median duration of exposure to a trial drug or placebo was 4 days (interquartile range, 3 to 7). The median number of days alive without delirium or coma was 8.5 (95% confidence interval [CI], 5.6 to 9.9) in the placebo group, 7.9 (95% CI, 4.4 to 9.6) in the haloperidol group, and 8.7 (95% CI, 5.9 to 10.0) in the ziprasidone group (P=0.26 for overall effect across trial groups). The use of haloperidol or ziprasidone, as compared with placebo, had no significant effect on the primary end point (odds ratios, 0.88 [95% CI, 0.64 to 1.21] and 1.04 [95% CI, 0.73 to 1.48], respectively). There were no significant between-group differences with respect to the secondary end points or the frequency of extrapyramidal symptoms.
Conclusions: The use of haloperidol or ziprasidone, as compared with placebo, in patients with acute respiratory failure or shock and hypoactive or hyperactive delirium in the ICU did not significantly alter the duration of delirium. (Funded by the National Institutes of Health and the VA Geriatric Research Education and Clinical Center; MIND-USA ClinicalTrials.gov number, NCT01211522 .).
Figures

Comment in
-
Dopamine Antagonists in ICU Delirium.N Engl J Med. 2018 Dec 27;379(26):2569-2570. doi: 10.1056/NEJMe1813382. Epub 2018 Oct 22. N Engl J Med. 2018. PMID: 30346241 No abstract available.
-
Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness.N Engl J Med. 2019 May 2;380(18):1778. doi: 10.1056/NEJMc1901272. N Engl J Med. 2019. PMID: 31042839 No abstract available.
-
Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness.N Engl J Med. 2019 May 2;380(18):1778-1779. doi: 10.1056/NEJMc1901272. N Engl J Med. 2019. PMID: 31042840 No abstract available.
-
Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness.N Engl J Med. 2019 May 2;380(18):1779. doi: 10.1056/NEJMc1901272. N Engl J Med. 2019. PMID: 31042841 No abstract available.
-
[Additive therapies : Intensive care studies from 2018-2019].Anaesthesist. 2020 Jan;69(1):52-54. doi: 10.1007/s00101-019-00642-3. Anaesthesist. 2020. PMID: 31444507 German. No abstract available.
References
-
- Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46(9):e825–e873. - PubMed
-
- Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
