Persistence of yellow fever virus-specific neutralizing antibodies after vaccination among US travellers
- PMID: 30346562
- PMCID: PMC7135922
- DOI: 10.1093/jtm/tay108
Persistence of yellow fever virus-specific neutralizing antibodies after vaccination among US travellers
Abstract
Background: Few studies have assessed the duration of humoral immunity following yellow fever (YF) vaccination in a non-endemic population. We evaluated seropositivity among US resident travellers based on time post-vaccination.
Methods: We identified serum samples from US travellers with YF virus-specific plaque reduction neutralization testing (PRNT) performed at CDC from 1988 to 2016. Analyses were conducted to assess the effect of time since vaccination on neutralizing antibody titer counts.
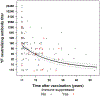
Results: Among 234 travellers who had neutralizing antibody testing performed on a specimen obtained ≥1 month after vaccination, 13 received multiple YF vaccinations and 221 had one dose of YF vaccine reported. All 13 who received more than one dose of YF vaccine had a positive PRNT regardless of the amount time since most recent vaccination. Among the 221 travellers with one reported dose of YF vaccine, 155 (70%) were vaccinated within 10 years (range 1 month-9 years) and 66 (30%) were vaccinated ≥10 years (range 10-53 years) prior to serum collection. Among the 155 individuals vaccinated, <10 years prior to serum collection, 146 (94%) had a positive PRNT compared with 82% (54/66) of individuals vaccinated ≥10 years prior to serum collection (P = 0.01). Post-vaccination PRNT titers showed a time-dependent decrease. Individuals with immunocompromising conditions were less likely to have a positive PRNT (77%) compared with those who were not immunocompromised (92%; P = 0.04).
Conclusion: Although the percentage of vaccinees with a positive PRNT and antibody titers decreased over time, a single dose of YF vaccine provided long-lasting protection in the majority of US travellers. A booster dose could be considered for certain travellers who are planning travel to a high risk area based on immune competence and time since vaccination.
Conflict of interest statement
Conflict of interest: None declared.
Figures
Comment in
-
Ten yearly yellow fever booster vaccinations may still be justified.J Travel Med. 2018 Jan 1;25(1). doi: 10.1093/jtm/tay130. J Travel Med. 2018. PMID: 30462249 No abstract available.
-
Waning immunity after single-dose yellow fever vaccination: Who needs a second shot?J Travel Med. 2019 Oct 14;26(7):tay134. doi: 10.1093/jtm/tay134. J Travel Med. 2019. PMID: 30476151 No abstract available.
-
Arguments for a two-dose yellow fever vaccination regimen in travellers.J Travel Med. 2019 Sep 2;26(6):taz004. doi: 10.1093/jtm/taz004. J Travel Med. 2019. PMID: 30657977 No abstract available.
References
-
- Staples JE, Monath TP, Gershman MD, Barrett AD. Yellow fever vaccines. In: Plotkin’s Vaccines 7th edition Philadelphia, PA: Saunders Elsevier, 2017.
-
- Centers for Disease Control and Prevention. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2010; 59:1–27. - PubMed
-
- World Health Organization. International Health Regulations, 3rd edn Geneva Switzerland: World Health Organization Press, 2005. Available at http://www.who.int/ihr/publications/9789241580496/en/ (17 July 2017, date last accessed).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

