Parvovirus B19V Nonstructural Protein NS1 Induces Double-Stranded Deoxyribonucleic Acid Autoantibodies and End-Organ Damage in Nonautoimmune Mice
- PMID: 30346568
- PMCID: PMC6468957
- DOI: 10.1093/infdis/jiy614
Parvovirus B19V Nonstructural Protein NS1 Induces Double-Stranded Deoxyribonucleic Acid Autoantibodies and End-Organ Damage in Nonautoimmune Mice
Abstract
Background: Viral infection is implicated in development of autoimmunity. Parvovirus B19 (B19V) nonstructural protein, NS1, a helicase, covalently modifies self double-stranded deoxyribonucleic acid (dsDNA) and induces apoptosis. This study tested whether resulting apoptotic bodies (ApoBods) containing virally modified dsDNA could induce autoimmunity in an animal model.
Methods: BALB/c mice were inoculated with (1) pristane-induced, (2) B19V NS1-induced, or (3) staurosporine-induced ApoBods. Serum was tested for dsDNA autoantibodies by Crithidia luciliae staining and enzyme-linked immunosorbent assay. Brain, heart, liver, and kidney pathology was examined. Deposition of self-antigens in glomeruli was examined by staining with antibodies to dsDNA, histones H1 and H4, and TATA-binding protein.
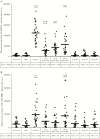
Results: The B19V NS1-induced ApoBod inoculation induced dsDNA autoantibodies in a dose-dependent fashion. Histopathological features of immune-mediated organ damage were evident in pristane-induced and NS1-induced ApoBod groups; severity scores were higher in these groups than in staurosporine-treated groups. Tissue damage was dependent on NS1-induced ApoBod dose. Nucleosomal antigens were deposited in target tissue from pristane-induced and NS1-induced ApoBod inoculated groups, but not in the staurosporine-induced ApoBod inoculated group.
Conclusions: This study demonstrated proof of principle in an animal model that virally modified dsDNA in apoptotic bodies could break tolerance to self dsDNA and induce dsDNA autoantibodies and end-organ damage.
Keywords: B19V; SLE; anti-dsDNA antibody; apoptotic bodies; glomerulonephritis.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




Similar articles
-
Human parvovirus B19 induced apoptotic bodies contain altered self-antigens that are phagocytosed by antigen presenting cells.PLoS One. 2013 Jun 12;8(6):e67179. doi: 10.1371/journal.pone.0067179. Print 2013. PLoS One. 2013. PMID: 23776709 Free PMC article.
-
The 11-Kilodalton Nonstructural Protein of Human Parvovirus B19 Facilitates Viral DNA Replication by Interacting with Grb2 through Its Proline-Rich Motifs.J Virol. 2018 Dec 10;93(1):e01464-18. doi: 10.1128/JVI.01464-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30282717 Free PMC article.
-
Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition.J Exp Med. 1998 Jul 6;188(1):29-38. doi: 10.1084/jem.188.1.29. J Exp Med. 1998. PMID: 9653081 Free PMC article.
-
Initiation of systemic autoimmunity and sequence specific anti-DNA autoantibodies.Crit Rev Immunol. 1999;19(2):117-26. Crit Rev Immunol. 1999. PMID: 10352900 Review.
-
Self-dsDNA in the pathogenesis of systemic lupus erythematosus.Clin Exp Immunol. 2018 Jan;191(1):1-10. doi: 10.1111/cei.13041. Epub 2017 Sep 15. Clin Exp Immunol. 2018. PMID: 28836661 Free PMC article. Review.
Cited by
-
Novel mutation N588 residue in the NS1 protein of feline parvovirus greatly augments viral replication.J Virol. 2024 May 14;98(5):e0009324. doi: 10.1128/jvi.00093-24. Epub 2024 Apr 9. J Virol. 2024. PMID: 38591899 Free PMC article.
-
Advances in the Development of Antiviral Strategies against Parvovirus B19.Viruses. 2019 Jul 18;11(7):659. doi: 10.3390/v11070659. Viruses. 2019. PMID: 31323869 Free PMC article. Review.
-
Double hit: Evans syndrome after malignant thymoma treatment and parvovirus B19 infection.BMJ Case Rep. 2020 Mar 18;13(3):e233485. doi: 10.1136/bcr-2019-233485. BMJ Case Rep. 2020. PMID: 32193179 Free PMC article.
-
Parvovirus B19 infection and kidney injury: report of 4 cases and analysis of immunization and viremia in an adult cohort of 100 patients undergoing a kidney biopsy.BMC Nephrol. 2020 Jul 9;21(1):260. doi: 10.1186/s12882-020-01911-9. BMC Nephrol. 2020. PMID: 32646497 Free PMC article.
-
The Relationship Between the Global Burden of Influenza From 2017 to 2019 and COVID-19: Descriptive Epidemiological Assessment.JMIR Public Health Surveill. 2021 Mar 2;7(3):e24696. doi: 10.2196/24696. JMIR Public Health Surveill. 2021. PMID: 33522974 Free PMC article.
References
-
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun 2010; 34:J168–77. - PubMed
-
- Cohen BJ, Buckley MM. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol 1988; 25:151–3. - PubMed
-
- Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya JM, Shoenfeld Y. Epstein-Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci 2007; 1108:567–77. - PubMed
-
- James JA, Escalante CR, Yoon-Robarts M, Edwards TA, Linden RM, Aggarwal AK. Crystal structure of the SF3 helicase from adeno-associated virus type 2. Structure 2003; 11:1025–35. - PubMed

