Sub-picomolar Inhibition of HIV-1 Protease with a Boronic Acid
- PMID: 30346745
- PMCID: PMC6249028
- DOI: 10.1021/jacs.8b07366
Sub-picomolar Inhibition of HIV-1 Protease with a Boronic Acid
Abstract
Boronic acids have been typecast as moieties for covalent complexation and are employed only rarely as agents for non-covalent recognition. By exploiting the profuse ability of a boronic acid group to form hydrogen bonds, we have developed an inhibitor of HIV-1 protease with extraordinary affinity. Specifically, we find that replacing an aniline moiety in darunavir with a phenylboronic acid leads to 20-fold greater affinity for the protease. X-ray crystallography demonstrates that the boronic acid group participates in three hydrogen bonds, more than the amino group of darunavir or any other analog. Importantly, the boronic acid maintains its hydrogen bonds and its affinity for the drug-resistant D30N variant of HIV-1 protease. The BOH···OC hydrogen bonds between the boronic acid hydroxy group and Asp30 (or Asn30) of the protease are short ( rO···O = 2.2 Å), and density functional theory analysis reveals a high degree of covalency. These data highlight the utility of boronic acids as versatile functional groups in the design of small-molecule ligands.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

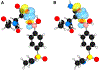
References
-
- Vondrasek J; Wlodawer A Annu. Rev. Biophys. Biomol. Struct 1998, 27, 249–284. - PubMed
- Ghosh AK, Ed. Aspartic Acid Proteases as Therapeutic Targets. Wiley–VCH: Weinheim, Germany, 2010.
-
- Prabu-Jeyabalan M; Nalivaika E; Schiffer CA Structure 2002, 10, 369–381. - PubMed
-
- Ripka AS; Rich DH Curr. Opin. Chem. Biol 1998, 2, 441–452. - PubMed
-
- Ghosh AK; Kincaid JF; Cho W; Walters DE; Krishnan K; Hussain KA; Koo Y; Cho H; Rudall C; Holland L; Buthod J Bioorg. Med. Chem. Lett 1998, 8, 687–90. - PubMed
-
- Koh Y; Nakata H; Maeda K; Ogata H; Bilcer G; Devasamudram T; Kincaid JF; Boross P; Wang YF; Tie Y; Volarath P; Gaddis L; Harrison RW; Weber IT; Ghosh AK; Mitsuya H Antimicrob. Agents Chemother 2003, 47, 3123–3129. - PMC - PubMed
- Ghosh AK; Dawson ZL; Mitsuya H Bioorg. Med. Chem 2007, 15, 7576–7580. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

