The microRNA-29/PGC1α regulatory axis is critical for metabolic control of cardiac function
- PMID: 30346946
- PMCID: PMC6211751
- DOI: 10.1371/journal.pbio.2006247
The microRNA-29/PGC1α regulatory axis is critical for metabolic control of cardiac function
Abstract
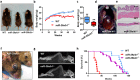
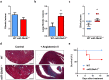
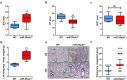
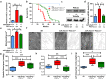
Different microRNAs (miRNAs), including miR-29 family, may play a role in the development of heart failure (HF), but the underlying molecular mechanisms in HF pathogenesis remain unclear. We aimed at characterizing mice deficient in miR-29 in order to address the functional relevance of this family of miRNAs in the cardiovascular system and its contribution to heart disease. In this work, we show that mice deficient in miR-29a/b1 develop vascular remodeling and systemic hypertension, as well as HF with preserved ejection fraction (HFpEF) characterized by myocardial fibrosis, diastolic dysfunction, and pulmonary congestion, and die prematurely. We also found evidence that the absence of miR-29 triggers the up-regulation of its target, the master metabolic regulator PGC1α, which in turn generates profound alterations in mitochondrial biogenesis, leading to a pathological accumulation of small mitochondria in mutant animals that contribute to cardiac disease. Notably, we demonstrate that systemic hypertension and HFpEF caused by miR-29 deficiency can be rescued by PGC1α haploinsufficiency, which reduces cardiac mitochondrial accumulation and extends longevity of miR-29-mutant mice. In addition, PGC1α is overexpressed in hearts from patients with HF. Collectively, our findings demonstrate the in vivo role of miR-29 in cardiovascular homeostasis and unveil a novel miR-29/PGC1α regulatory circuitry of functional relevance for cell metabolism under normal and pathological conditions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

