Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial
- PMID: 30347025
- PMCID: PMC6439843
- DOI: 10.1001/jamaoncol.2018.5152
Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial
Abstract
Importance: The humanized monoclonal antibody atezolizumab targets programmed death-ligand 1 and has demonstrated durable single-agent activity in a subset of metastatic triple-negative breast cancers. To extend the observed activity, combinatorial approaches are being tested with standard cytotoxic chemotherapies known to induce immunogenic tumor cell death.
Objective: To examine the safety, tolerability, and preliminary clinical activity of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancers.
Design, setting, and participants: This phase 1b multicohort study enrolled 33 women with stage IV or locally recurrent triple-negative breast cancers and 0 to 2 lines of prior chemotherapy in the metastatic setting from December 8, 2014, to April 30, 2017, at 11 sites in the United States. The median follow-up was 24.4 months (95% CI, 22.1-28.8 months).
Interventions: Patients received concurrent intravenous atezolizumab and intravenous nab-paclitaxel (minimum 4 cycles).
Main outcomes and measures: The primary end point was safety and tolerability. Secondary end points included best overall response rate by Response Evaluation Criteria in Solid Tumors, version 1.1; objective response rate; duration of response; disease control rate; progression-free survival; overall survival; and biomarker analyses.
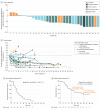
Results: The 33 women had a median age of 55 years (range, 32-84 years) and received 1 or more doses of atezolizumab. All patients (100%) experienced at least 1 treatment-related adverse event, 24 patients (73%) experienced grade 3/4 adverse events, and 7 patients (21%) had grade 3/4 adverse events of special interest. No deaths were related to study treatment. The objective response rate was 39.4% (95% CI, 22.9%-57.9%), and the median duration of response was 9.1 months (95% CI, 2.0-20.9 months). The disease control rate was 51.5% (95% CI, 33.5%-69.2%). Median progression-free survival and overall survival were 5.5 months (95% CI, 5.1-7.7 months) and 14.7 months (95% CI, 10.1-not estimable), respectively. Concurrent nab-paclitaxel neither significantly changed biomarkers of the tumor immune microenvironment (programmed death-ligand 1, tumor-infiltrating lymphocytes, CD8) nor impaired atezolizumab systemic immune activation (expansion of proliferating CD8+ T cells, increase of CXCL10 chemokine).
Conclusions and relevance: In this phase 1b trial for metastatic triple-negative breast cancers, the combination of atezolizumab plus nab-paclitaxel had a manageable safety profile. Antitumor responses were observed, including in patients previously treated with a taxane.
Trial registration: ClinicalTrials.gov identifier: NCT01633970.
Conflict of interest statement
Figures
Comment in
-
Concerns Regarding Phase 1b Clinical Trial of Atezolizumab Plus Nab-Paclitaxel for Metastatic Breast Cancer-In Reply.JAMA Oncol. 2019 Jun 1;5(6):908-909. doi: 10.1001/jamaoncol.2019.0317. JAMA Oncol. 2019. PMID: 30998805 No abstract available.
-
Concerns Regarding Phase 1b Clinical Trial of Atezolizumab Plus nab-Paclitaxel for Metastatic Breast Cancer.JAMA Oncol. 2019 Jun 1;5(6):908. doi: 10.1001/jamaoncol.2019.0314. JAMA Oncol. 2019. PMID: 30998806 No abstract available.
Similar articles
-
Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol. 2020 Jan;21(1):44-59. doi: 10.1016/S1470-2045(19)30689-8. Epub 2019 Nov 27. Lancet Oncol. 2020. PMID: 31786121 Clinical Trial.
-
Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer.N Engl J Med. 2018 Nov 29;379(22):2108-2121. doi: 10.1056/NEJMoa1809615. Epub 2018 Oct 20. N Engl J Med. 2018. PMID: 30345906 Clinical Trial.
-
Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial.Lancet. 2020 Oct 10;396(10257):1090-1100. doi: 10.1016/S0140-6736(20)31953-X. Epub 2020 Sep 20. Lancet. 2020. PMID: 32966830 Clinical Trial.
-
Atezolizumab (in Combination with Nab-Paclitaxel): A Review in Advanced Triple-Negative Breast Cancer.Drugs. 2020 Apr;80(6):601-607. doi: 10.1007/s40265-020-01295-y. Drugs. 2020. PMID: 32248356 Review.
-
Atezolizumab for use in PD-L1-positive unresectable, locally advanced or metastatic triple-negative breast cancer.Future Oncol. 2020 Jan;16(3):4439-4453. doi: 10.2217/fon-2019-0468. Epub 2019 Dec 12. Future Oncol. 2020. PMID: 31829043 Review.
Cited by
-
An overview of immune checkpoint inhibitors in breast cancer.Explor Target Antitumor Ther. 2020;1(6):452-472. doi: 10.37349/etat.2020.00029. Epub 2020 Dec 28. Explor Target Antitumor Ther. 2020. PMID: 36046385 Free PMC article. Review.
-
Subgroup analysis of Japanese patients in a Phase 3 study of atezolizumab in advanced triple-negative breast cancer (IMpassion130).Jpn J Clin Oncol. 2019 Dec 27;49(12):1083-1091. doi: 10.1093/jjco/hyz135. Jpn J Clin Oncol. 2019. PMID: 31612909 Free PMC article. Clinical Trial.
-
Harnessing DNA Repair Defects to Augment Immune-Based Therapies in Triple-Negative Breast Cancer.Front Oncol. 2021 Sep 24;11:703802. doi: 10.3389/fonc.2021.703802. eCollection 2021. Front Oncol. 2021. PMID: 34631532 Free PMC article. Review.
-
Synthesis and Optimization of the Docetaxel-Loaded and Durvalumab-Targeted Human Serum Albumin Nanoparticles, In Vitro Characterization on Triple-Negative Breast Cancer Cells.ACS Omega. 2023 Jul 13;8(29):26287-26300. doi: 10.1021/acsomega.3c02682. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521641 Free PMC article.
-
Atezolizumab and paclitaxel as first line therapy in advanced triple-negative breast cancer patients included in the French early access program.Sci Rep. 2023 Aug 18;13(1):13427. doi: 10.1038/s41598-023-40569-9. Sci Rep. 2023. PMID: 37596388 Free PMC article.
References
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed March 20, 2018.
-
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860-867. doi: 10.1200/JCO.2011.41.0902 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

