Clinical Features of a Retinopathy Associated With a Dominant Allele of the RGR Gene
- PMID: 30347075
- PMCID: PMC6181194
- DOI: 10.1167/iovs.18-25061
Clinical Features of a Retinopathy Associated With a Dominant Allele of the RGR Gene
Abstract
Purpose: We describe the clinical features in two pedigrees with dominantly inherited retinopathy segregating the previously reported frameshifting mutation, c.836dupG (p.Ile280Asn*78) in the terminal exon of the RGR gene, and compare their haplotypes to that of the previously reported pedigree.
Methods: The probands were ascertained at West Virginia University Eye Institute (WVU) and Moorfields Eye Hospital (MEH) through next generation sequencing (NGS) and whole genome sequencing (WGS) respectively. Clinical data included visual acuity (VA), visual fields, fundus autofluorescence (FAF), optical coherence tomography (OCT), and electroretinography (ERG). Haplotype analysis was performed using Sanger sequencing of the DNA from the molecularly ascertained individuals from the three pedigrees.
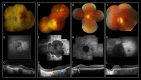
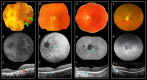
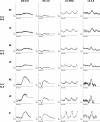
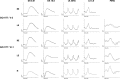
Results: Nine heterozygous mutation carriers were identified in two families. Four carriers were asymptomatic; five carriers had variable VA reduction, visual field constriction, and experienced difficulty under dim illumination. Fundus examination of the asymptomatic carriers showed diffuse or reticular pigmentation of the retina; the symptomatic carriers had chorioretinal atrophy. FAF imaging showed widespread signal loss in advanced retinopathy, and reticular hyperautofluorescence in mild cases. OCT showed loss of outer retinal lamina in advanced disease. ERG showed moderate-to-severe rod-cone dysfunction in two symptomatic carriers; and was normal in three asymptomatic carriers. A shared haplotype flanking the mutation of up to 6.67 Mb was identified in both families. Within this region, 1.27 Mb were shared with the first family reported with this retinopathy.
Conclusions: The clinical data suggest a variable and slow degeneration of the RPE. A shared chromosomal segment surrounding the RGR gene suggests a single ancestral mutational event underlying all three families.
Figures
References
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–323. - PubMed
-
- Dryja TP, McGee TL, Reichel E, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. - PubMed
-
- Jiang M, Pandey S, Fong HK. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34:3669–3678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

