Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging
- PMID: 30347683
- PMCID: PMC6316340
- DOI: 10.3390/bios8040095
Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging
Abstract
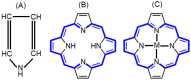
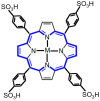
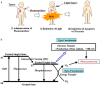
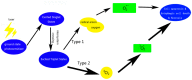
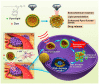
In recent years, scientific advancements have constantly increased at a significant rate in the field of biomedical science. Keeping this in view, the application of porphyrins and metalloporphyrins in the field of biomedical science is gaining substantial importance. Porphyrins are the most widely studied tetrapyrrole-based compounds because of their important roles in vital biological processes. The cavity of porphyrins containing four pyrrolic nitrogens is well suited for the binding majority of metal ions to form metalloporphyrins. Porphyrins and metalloporphyrins possess peculiar photochemical, photophysical, and photoredox properties which are tunable through structural modifications. Their beneficial photophysical properties, such as the long wavelength of emission and absorption, high singlet oxygen quantum yield, and low in vivo toxicity, have drawn scientists' interest to discover new dimensions in the biomedical field. Applications of porphyrins and metalloporphyrins have been pursued in the perspective of contrast agents for magnetic resonance imaging (MRI), photodynamic therapy (PDT) of cancer, bio-imaging, and other biomedical applications. This review discusses photophysics and the photochemistry of porphyrins and their metal complexes. Secondly, it explains the current developments and mode of action for contrast agents for MRI. Moreover, the application of porphyrin and metalloporphyrin-based molecules as a photosensitizer in PDT of cancer, the mechanism of the generation of reactive oxygen species (ROS), factors that determine the efficiency of PDT, and the developments to improve this technology are delineated. The last part explores the most recent research and developments on metalloporphyrin-based materials in bio-imaging, drug delivery, and the determination of ferrochelatase in bone marrow indicating their prospective clinical applications.
Keywords: bio-imaging; contrast agents; drug delivery; magnetic resonance imaging; metalloporphyrins; photochemistry; photodynamic therapy; photophysics; photosensitizer; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Valicsek Z., Horváth O. Application of the electronic spectra of porphyrins for analytical purposes: The effects of metal ions and structural distortions. Microchem. J. 2013;107:47–62. doi: 10.1016/j.microc.2012.07.002. - DOI
-
- Valicsek Z., Horváth O., Lendvay G., Kikaš I., Škorić I. Formation, photophysics, and photochemistry of cadmium(II) complexes with 5, 10, 15, 20-tetrakis (4-sulfonatophenyl) porphyrin and its octabromo derivative: The effects of bromination and the axial hydroxo ligand. J. Photochem. Photobiol. A Chem. 2011;218:143–155. doi: 10.1016/j.jphotochem.2010.12.014. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

