The Proposal of Molecular Mechanisms of Weak Organic Acids Intake-Induced Improvement of Insulin Resistance in Diabetes Mellitus via Elevation of Interstitial Fluid pH
- PMID: 30347717
- PMCID: PMC6214001
- DOI: 10.3390/ijms19103244
The Proposal of Molecular Mechanisms of Weak Organic Acids Intake-Induced Improvement of Insulin Resistance in Diabetes Mellitus via Elevation of Interstitial Fluid pH
Abstract
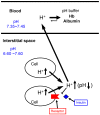
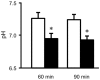
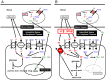
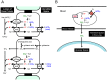
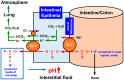
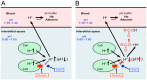
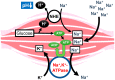
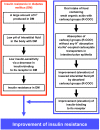
Blood contains powerful pH-buffering molecules such as hemoglobin (Hb) and albumin, while interstitial fluids have little pH-buffering molecules. Thus, even under metabolic disorder conditions except severe cases, arterial blood pH is kept constant within the normal range (7.35~7.45), but the interstitial fluid pH under metabolic disorder conditions becomes lower than the normal level. Insulin resistance is one of the most important key factors in pathogenesis of diabetes mellitus, nevertheless the molecular mechanism of insulin resistance occurrence is still unclear. Our studies indicate that lowered interstitial fluid pH occurs in diabetes mellitus, causing insulin resistance via reduction of the binding affinity of insulin to its receptor. Therefore, the key point for improvement of insulin resistance occurring in diabetes mellitus is development of methods or techniques elevating the lowered interstitial fluid pH. Intake of weak organic acids is found to improve the insulin resistance by elevating the lowered interstitial fluid pH in diabetes mellitus. One of the molecular mechanisms of the pH elevation is that: (1) the carboxyl group (R-COO-) but not H⁺ composing weak organic acids in foods is absorbed into the body, and (2) the absorbed the carboxyl group (R-COO-) behaves as a pH buffer material, elevating the interstitial fluid pH. On the other hand, high salt intake has been suggested to cause diabetes mellitus; however, the molecular mechanism is unclear. A possible mechanism of high salt intake-caused diabetes mellitus is proposed from a viewpoint of regulation of the interstitial fluid pH: high salt intake lowers the interstitial fluid pH via high production of H⁺ associated with ATP synthesis required for the Na⁺,K⁺-ATPase to extrude the high leveled intracellular Na⁺ caused by high salt intake. This review article introduces the molecular mechanism causing the lowered interstitial fluid pH and insulin resistance in diabetes mellitus, the improvement of insulin resistance via intake of weak organic acid-containing foods, and a proposal mechanism of high salt intake-caused diabetes mellitus.
Keywords: alkalization; binding affinity; food; insulin; interstitial fluid; pH; weak organic acid.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

