Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer
- PMID: 30347768
- PMCID: PMC6222505
- DOI: 10.3390/molecules23102703
Cationic Cyclopentadienyliron Complex as a Novel and Successful Nucleating Agent on the Crystallization Behavior of the Biodegradable PHB Polymer
Abstract
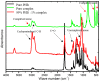
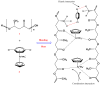
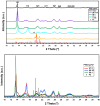
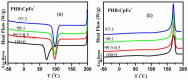
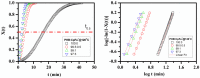
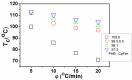
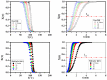
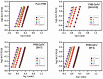
Cationic cyclopentadienyliron (CpFe⁺) is one of the most fruitful organometallic moieties that has been utilized to mediate the facile synthesis of a massive number of macromolecules. However, the ability of this compound to function as a nucleating agent to improve other macromolecule properties has not been explored. This report scrutinizes the influence of the cationic complex as a novel nucleating agent on the spherulitic morphology, crystal structure, and isothermal and non-isothermal crystallization behavior of the Poly(3-hydroxybutyrate) (PHB) bacterial origin. The incorporation of the CpFe⁺ into the PHB materials caused a significant increase in its spherulitic numbers with a remarkable reduction in the spherulitic sizes. Unlike other nucleating agents, the SEM imageries exhibited a good dispersion without forming agglomerates of the CpFe⁺ moieties in the PHB matrix. Moreover, according to the FTIR analysis, the cationic organoiron complex has a strong interaction with the PHB polymeric chains via the coordination with its ester carbonyl. Yet, the XRD results revealed that this incorporation had no significant effect on the PHB crystalline structure. Though the CpFe⁺ had no effect on the polymer's crystal structure, it accelerated outstandingly the melt crystallization of the PHB. Meanwhile, the crystallization half-times (t0.5) of the PHB decreased dramatically with the addition of the CpFe⁺. The isothermal and non-isothermal crystallization processes were successfully described using the Avrami model and a modified Avrami model, as well as a combination of the Avrami and Ozawa methods. Finally, the effective activation energy of the PHB/CpFe⁺ nanocomposites was much lower than those of their pure counterparts, which supported the heterogeneous nucleation mechanism with the organometallic moieties, indicating that the CpFe⁺ is a superior nucleating agent for this class of polymer.
Keywords: FTIR analysis; bacterial Poly(3-hydroxybutyrate) (PHB); cationic organoiron complex; crystal structure; isothermal and non-isothermal crystallization; nanocomposites; spherulitic morphology; thermal stability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Crystallization kinetics and thermal property of biodegradable poly(3-hydroxybutyrate)/graphene oxide nanocomposites.J Nanosci Nanotechnol. 2012 Sep;12(9):7314-21. doi: 10.1166/jnn.2012.6461. J Nanosci Nanotechnol. 2012. PMID: 23035470
-
New biodegradable polyhydroxybutyrate/layered silicate nanocomposites.Biomacromolecules. 2007 Nov;8(11):3393-400. doi: 10.1021/bm700500t. Epub 2007 Oct 25. Biomacromolecules. 2007. PMID: 17958439
-
Selective localization of starch nanocrystals in the biodegradable nanocomposites probed by crystallization temperatures.Carbohydr Polym. 2020 Jan 1;227:115341. doi: 10.1016/j.carbpol.2019.115341. Epub 2019 Sep 19. Carbohydr Polym. 2020. PMID: 31590874
-
Biotechnological production of (R)-3-hydroxybutyric acid monomer.J Biotechnol. 2007 Nov 1;132(3):264-72. doi: 10.1016/j.jbiotec.2007.03.015. Epub 2007 Apr 22. J Biotechnol. 2007. PMID: 17543411 Review.
-
Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications.Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:1092-1116. doi: 10.1016/j.msec.2017.11.006. Epub 2017 Nov 13. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30184731 Review.
Cited by
-
Synergistic effect of TiO2 nanoparticles and poly (ethylene-co-vinyl acetate) on the morphology and crystallization behavior of polylactic acid.Sci Rep. 2024 Aug 5;14(1):18142. doi: 10.1038/s41598-024-68023-4. Sci Rep. 2024. PMID: 39103411 Free PMC article.
-
Biobased Poly(ethylene furanoate) Polyester/TiO₂ Supported Nanocomposites as Effective Photocatalysts for Anti-inflammatory/Analgesic Drugs.Molecules. 2019 Feb 4;24(3):564. doi: 10.3390/molecules24030564. Molecules. 2019. PMID: 30720725 Free PMC article.
-
Morphology and thermal properties of poly(l-lactic acid) nucleated with 2,2'-(butane-1,4-diylbis(oxy))di(benzohydrazide).RSC Adv. 2025 Apr 28;15(17):13539-13551. doi: 10.1039/d5ra00368g. eCollection 2025 Apr 22. RSC Adv. 2025. PMID: 40296991 Free PMC article.
References
-
- Bugnicourt E., Cinelli P., Lazzeri A., Alvarez V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express. Polym. Lett. 2014;8:791–808. doi: 10.3144/expresspolymlett.2014.82. - DOI
-
- Gumel A.M., Annuar M.S.M., Chisti Y. Recent Advances in the Production, Recovery and Applications of Polyhydroxyalkanoates. J. Polym. Environ. 2013;21:580–605. doi: 10.1007/s10924-012-0527-1. - DOI
-
- Vilela C., Sousa A.F., Fonseca A.C., Serra A.C., Coelho J.F.J., Freire C.S.R., Silvestre A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014;5:3119–3141. doi: 10.1039/C3PY01213A. - DOI
-
- Lagarón J.M., López-Rubio A., José Fabra M. Bio-based packaging. J. Appl. Polym. Sci. 2016;133 doi: 10.1002/app.42971. - DOI
-
- Pan Y., Farmahini-Farahani M., O’Hearn P., Xiao H., Ocampo H. An overview of bio-based polymers for packaging materials. J. Bioresour. Bioprod. 2016;1:106–113.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

