Tuning the Anti(myco)bacterial Activity of 3-Hydroxy-4-pyridinone Chelators through Fluorophores
- PMID: 30347802
- PMCID: PMC6316862
- DOI: 10.3390/ph11040110
Tuning the Anti(myco)bacterial Activity of 3-Hydroxy-4-pyridinone Chelators through Fluorophores
Abstract
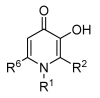
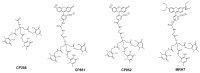
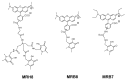
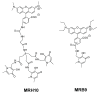
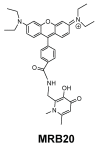
Controlling the sources of Fe available to pathogens is one of the possible strategies that can be successfully used by novel antibacterial drugs. We focused our interest on the design of chelators to address Mycobacterium avium infections. Taking into account the molecular structure of mycobacterial siderophores and considering that new chelators must be able to compete for Fe(III), we selected ligands of the 3-hydroxy-4-pyridinone class to achieve our purpose. After choosing the type of chelating unit it was also our objective to design chelators that could be monitored inside the cell and for that reason we designed chelators that could be functionalized with fluorophores. We didn't realize at the time that the incorporation a fluorophore, to allow spectroscopic detection, would be so relevant for the antimycobacterial effect or to determine the affinity of the chelators towards biological membranes. From a biophysical perspective, this is a fascinating illustration of the fact that functionalization of a molecule with a particular label may lead to a change in its membrane permeation properties and result in a dramatic change in biological activity. For that reason we believe it is interesting to give a critical account of our entire work in this area and justify the statement "to label means to change". New perspectives regarding combined therapeutic approaches and the use of rhodamine B conjugates to target closely related problems such as bacterial resistance and biofilm production are also discussed.
Keywords: 3-hydroxy-4-pyridinone; antibacterial activity; bacteria; fluorescent iron chelator; fluorophore; membrane interactions; rhodamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
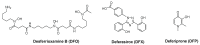


References
-
- Bertini P.I., Bertini I., Gray H.B., Gray P.H., Stiefel E.I., Stiefel P.E., Valentine J.S. Biological Inorganic Chemistry: Structure and Reactivity (Tutorial II—Fundamentals of Coordination Chemistry) 1st ed. University Science Books; Sausalito, CA, USA: 2007.
-
- Crichton R. Iron Metabolism: From Molecular Mechanisms to Clinical Consequences. 3rd ed. John Wiley & Sons Ltd.; Chichester, West Sussex, UK: 2009.
-
- Archibald F. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol. Lett. 1983;19:29–32. doi: 10.1111/j.1574-6968.1983.tb00504.x. - DOI
Publication types
LinkOut - more resources
Full Text Sources

