Cytoprotective Effects of Natural Compounds against Oxidative Stress
- PMID: 30347819
- PMCID: PMC6210295
- DOI: 10.3390/antiox7100147
Cytoprotective Effects of Natural Compounds against Oxidative Stress
Abstract
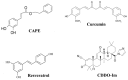
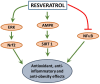
Oxidative stress, an imbalance between reactive oxygen species and antioxidants, has been witnessed in pathophysiological states of many disorders. Compounds identified from natural sources have long been recognized to ameliorate oxidative stress due to their inherent antioxidant activities. Here, we summarize the cytoprotective effects and mechanisms of natural or naturally derived synthetic compounds against oxidative stress. These compounds include: caffeic acid phenethyl ester (CAPE) found in honey bee propolis, curcumin from turmeric roots, resveratrol abundant in grape, and 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole (CDDO-Im), a synthetic triterpenoid based on naturally occurring oleanolic acid. Cytoprotective effects of these compounds in diseases conditions like cardiovascular diseases and obesity to decrease oxidative stress are discussed.
Keywords: CAPE; CDDO-Im; cardiovascular diseases; curcumin; cytoprotection; obesity; oxidative stress; resveratrol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Cytoprotection of human endothelial cells against oxidative stress by 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im): application of systems biology to understand the mechanism of action.Eur J Pharmacol. 2014 Jul 5;734:122-31. doi: 10.1016/j.ejphar.2014.03.033. Epub 2014 Apr 3. Eur J Pharmacol. 2014. PMID: 24703885
-
Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress.Molecules. 2022 Jul 8;27(14):4396. doi: 10.3390/molecules27144396. Molecules. 2022. PMID: 35889266 Free PMC article. Review.
-
Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole.Cancer Res. 2006 Feb 15;66(4):2488-94. doi: 10.1158/0008-5472.CAN-05-3823. Cancer Res. 2006. PMID: 16489057
-
Time Course Expression Analysis of 1[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole Induction of Cytoprotection in Human Endothelial Cells.Gene Regul Syst Bio. 2017 Apr 7;11:1177625017701106. doi: 10.1177/1177625017701106. eCollection 2017. Gene Regul Syst Bio. 2017. PMID: 28469413 Free PMC article.
-
Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-reperfusion Injury and Drug Adverse Reactions.Crit Rev Food Sci Nutr. 2016 Oct 2;56(13):2183-90. doi: 10.1080/10408398.2013.821967. Crit Rev Food Sci Nutr. 2016. PMID: 25365228 Review.
Cited by
-
Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.).Int J Mol Sci. 2020 Jan 8;21(2):397. doi: 10.3390/ijms21020397. Int J Mol Sci. 2020. PMID: 31936315 Free PMC article.
-
Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway.Antioxidants (Basel). 2020 Feb 1;9(2):121. doi: 10.3390/antiox9020121. Antioxidants (Basel). 2020. PMID: 32024207 Free PMC article.
-
Eat4Genes: a bioinformatic rational gene targeting app and prototype model for improving human health.Front Nutr. 2023 May 26;10:1196520. doi: 10.3389/fnut.2023.1196520. eCollection 2023. Front Nutr. 2023. PMID: 37305078 Free PMC article.
-
UPLC-MS metabolite profiling and antioxidant activity of Sanghuangporus sanghuang extract.PeerJ. 2025 Jan 22;13:e18758. doi: 10.7717/peerj.18758. eCollection 2025. PeerJ. 2025. PMID: 39866569 Free PMC article.
-
Metallo-protease Peptidase M84 from Bacillusaltitudinis induces ROS-dependent apoptosis in ovarian cancer cells by targeting PAR-1.iScience. 2024 Apr 26;27(6):109828. doi: 10.1016/j.isci.2024.109828. eCollection 2024 Jun 21. iScience. 2024. PMID: 38799586 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

