mTOR Complexes as a Nutrient Sensor for Driving Cancer Progression
- PMID: 30347859
- PMCID: PMC6214109
- DOI: 10.3390/ijms19103267
mTOR Complexes as a Nutrient Sensor for Driving Cancer Progression
Abstract
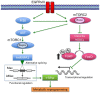
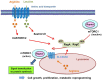
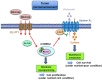
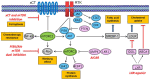
Recent advancement in the field of molecular cancer research has clearly revealed that abnormality of oncogenes or tumor suppressor genes causes tumor progression thorough the promotion of intracellular metabolism. Metabolic reprogramming is one of the strategies for cancer cells to ensure their survival by enabling cancer cells to obtain the macromolecular precursors and energy needed for the rapid growth. However, an orchestration of appropriate metabolic reactions for the cancer cell survival requires the precise mechanism to sense and harness the nutrient in the microenvironment. Mammalian/mechanistic target of rapamycin (mTOR) complexes are known downstream effectors of many cancer-causing mutations, which are thought to regulate cancer cell survival and growth. Recent studies demonstrate the intriguing role of mTOR to achieve the feat through metabolic reprogramming in cancer. Importantly, not only mTORC1, a well-known regulator of metabolism both in normal and cancer cell, but mTORC2, an essential partner of mTORC1 downstream of growth factor receptor signaling, controls cooperatively specific metabolism, which nominates them as an essential regulator of cancer metabolism as well as a promising candidate to garner and convey the nutrient information from the surrounding environment. In this article, we depict the recent findings on the role of mTOR complexes in cancer as a master regulator of cancer metabolism and a potential sensor of nutrients, especially focusing on glucose and amino acid sensing in cancer. Novel and detailed molecular mechanisms that amino acids activate mTOR complexes signaling have been identified. We would also like to mention the intricate crosstalk between glucose and amino acid metabolism that ensures the survival of cancer cells, but at the same time it could be exploitable for the novel intervention to target the metabolic vulnerabilities of cancer cells.
Keywords: cancer; mTOR complex; metabolic reprogramming; microenvironment; nutrient sensor.
Conflict of interest statement
P.S.M. is a scientific co-founder and consultant for Pretzel Therapeutics, Inc.
Figures
Similar articles
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
Regulation and metabolic functions of mTORC1 and mTORC2.Physiol Rev. 2021 Jul 1;101(3):1371-1426. doi: 10.1152/physrev.00026.2020. Epub 2021 Feb 18. Physiol Rev. 2021. PMID: 33599151 Free PMC article. Review.
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
-
Integrated actions of mTOR complexes 1 and 2 for growth and development of Dictyostelium.Int J Dev Biol. 2019;63(8-9-10):521-527. doi: 10.1387/ijdb.190245ak. Int J Dev Biol. 2019. PMID: 31840789 Review.
-
mTORC2 Signaling: A Path for Pancreatic β Cell's Growth and Function.J Mol Biol. 2018 Mar 30;430(7):904-918. doi: 10.1016/j.jmb.2018.02.013. Epub 2018 Feb 23. J Mol Biol. 2018. PMID: 29481838 Review.
Cited by
-
The metabolomic landscape plays a critical role in glioma oncogenesis.Cancer Sci. 2022 May;113(5):1555-1563. doi: 10.1111/cas.15325. Epub 2022 Mar 23. Cancer Sci. 2022. PMID: 35271755 Free PMC article. Review.
-
Targeting mTOR in Acute Lymphoblastic Leukemia.Cells. 2019 Feb 21;8(2):190. doi: 10.3390/cells8020190. Cells. 2019. PMID: 30795552 Free PMC article. Review.
-
Amino Acid Transporter SLC6A14 (ATB0,+) - A Target in Combined Anti-cancer Therapy.Front Cell Dev Biol. 2020 Oct 21;8:594464. doi: 10.3389/fcell.2020.594464. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195271 Free PMC article. Review.
-
mTOR in Human Diseases.Int J Mol Sci. 2019 May 11;20(9):2351. doi: 10.3390/ijms20092351. Int J Mol Sci. 2019. PMID: 31083592 Free PMC article.
-
Causes and Consequences of Variable Tumor Cell Metabolism on Heritable Modifications and Tumor Evolution.Front Oncol. 2020 Mar 27;10:373. doi: 10.3389/fonc.2020.00373. eCollection 2020. Front Oncol. 2020. PMID: 32292719 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

