PG-Priming Enhances Doxorubicin Influx to Trigger Necrotic and Autophagic Cell Death in Oral Squamous Cell Carcinoma
- PMID: 30347872
- PMCID: PMC6210351
- DOI: 10.3390/jcm7100375
PG-Priming Enhances Doxorubicin Influx to Trigger Necrotic and Autophagic Cell Death in Oral Squamous Cell Carcinoma
Abstract
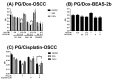
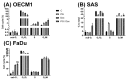
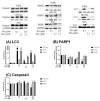
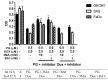
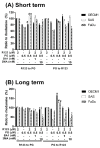
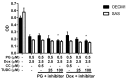
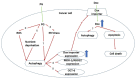
Synergistic effects between natural compounds and chemotherapy drugs are believed to have fewer side effects with equivalent efficacy. However, the synergistic potential of prodigiosin (PG) with doxorubicin (Dox) chemotherapy is still unknown. This study explores the synergistic mechanism of PG and Dox against oral squamous cell carcinoma (OSCC) cells. Three OSCC cell lines were treated with different PG/Dox combinatory schemes for cytotoxicity tests and were further investigated for cell death characteristics by cell cycle flow cytometry and autophagy/apoptosis marker labelling. When OSCC cells were pretreated with PG, the cytotoxicity of the subsequent Dox-treatment was 30% higher than Dox alone. The cytotoxic efficacy of PG-pretreated was found better than those of PG plus Dox co-treatment and Dox-pretreatment. Increase of Sub-G1 phase and caspase-3/LC-3 levels without poly (ADP-ribose) polymeras (PARP) elevation indicated both autophagy and necrosis occurred in OSCC cells. Dox flux after PG-priming was further evaluated by rhodamine-123 accumulation and Dox transporters analysis to elucidate the PG-priming effect. PG-priming autophagy enhanced Dox accumulation according to the increase of rhodamine-123 accumulation without the alterations of Dox transporters. Additionally, the cause of PG-triggered autophagy was determined by co-treatment with endoplasmic reticulum (ER) stress or AMP-activated protein kinase (AMPK) inhibitor. PG-induced autophagy was not related to nutrient deprivation and ER stress was proved by co-treatment with specific inhibitor. Taken together, PG-priming autophagy could sensitize OSCC cells by promoting Dox influx without regulation of Dox transporter. The PG-priming might be a promising adjuvant approach for the chemotherapy of OSCC.
Keywords: autophagy; doxorubicin; influx; priming; prodigiosin.
Conflict of interest statement
The authors declare no any conflict of interest.
Figures

Similar articles
-
Doxorubicin metabolism moderately attributes to putative toxicity in prodigiosin/doxorubicin synergism in vitro cells.Mol Cell Biochem. 2020 Dec;475(1-2):119-126. doi: 10.1007/s11010-020-03864-x. Epub 2020 Aug 4. Mol Cell Biochem. 2020. PMID: 32754875 Free PMC article.
-
Prodigiosin-Emerged PI3K/Beclin-1-Independent Pathway Elicits Autophagic Cell Death in Doxorubicin-Sensitive and -Resistant Lung Cancer.J Clin Med. 2018 Oct 3;7(10):321. doi: 10.3390/jcm7100321. J Clin Med. 2018. PMID: 30282915 Free PMC article.
-
Isomahanine induces endoplasmic reticulum stress and simultaneously triggers p38 MAPK-mediated apoptosis and autophagy in multidrug-resistant human oral squamous cell carcinoma cells.Oncol Rep. 2017 Feb;37(2):1243-1252. doi: 10.3892/or.2017.5352. Epub 2017 Jan 4. Oncol Rep. 2017. PMID: 28075474
-
Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells.Int J Mol Sci. 2017 May 12;18(5):1048. doi: 10.3390/ijms18051048. Int J Mol Sci. 2017. PMID: 28498322 Free PMC article.
-
Inhibitory Growth of Oral Squamous Cell Carcinoma Cancer via Bacterial Prodigiosin.Mar Drugs. 2017 Jul 15;15(7):224. doi: 10.3390/md15070224. Mar Drugs. 2017. PMID: 28714874 Free PMC article.
Cited by
-
Pigment production by cold-adapted bacteria and fungi: colorful tale of cryosphere with wide range applications.Extremophiles. 2020 Jul;24(4):447-473. doi: 10.1007/s00792-020-01180-2. Epub 2020 Jun 1. Extremophiles. 2020. PMID: 32488508 Free PMC article. Review.
-
Bacterial pigments: A colorful palette reservoir for biotechnological applications.Biotechnol Appl Biochem. 2022 Jun;69(3):981-1001. doi: 10.1002/bab.2170. Epub 2021 May 2. Biotechnol Appl Biochem. 2022. PMID: 33870552 Free PMC article. Review.
-
Production and Potential Applications of Bioconversion of Chitin and Protein-Containing Fishery Byproducts into Prodigiosin: A Review.Molecules. 2020 Jun 13;25(12):2744. doi: 10.3390/molecules25122744. Molecules. 2020. PMID: 32545769 Free PMC article. Review.
-
Prodigiosin Sensitizes Sensitive and Resistant Urothelial Carcinoma Cells to Cisplatin Treatment.Molecules. 2021 Feb 27;26(5):1294. doi: 10.3390/molecules26051294. Molecules. 2021. PMID: 33673611 Free PMC article.
-
Prodigiosin from Serratia Marcescens in Cockroach Inhibits the Proliferation of Hepatocellular Carcinoma Cells through Endoplasmic Reticulum Stress-Induced Apoptosis.Molecules. 2022 Oct 26;27(21):7281. doi: 10.3390/molecules27217281. Molecules. 2022. PMID: 36364107 Free PMC article.
References
-
- Johnson-Arbor K., Dubey R. StatPearls. StatPearls Publisher; Treasure Island, FL, USA: 2018. Doxorubicin.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

